On the premise of ensuring the authenticity and reliability of electronic prescription sources, online sales of prescription drugs are allowed.
Editor’s note: This article is from the WeChat public account “arterial network” (ID: vcbeat) , author: Liuzong Yu.
During this year’s epidemic, Internet medical care took full advantage of the Internet. Online diagnosis and treatment, out-of-prescription prescriptions, medical insurance payment, and home delivery of medicines all played a major role, facilitating medical treatment and drug purchase. In the past six months, Internet medical treatment has ushered in continuous and intensive policy encouragement, and the combination of Internet and medical treatment has become more and more deep.
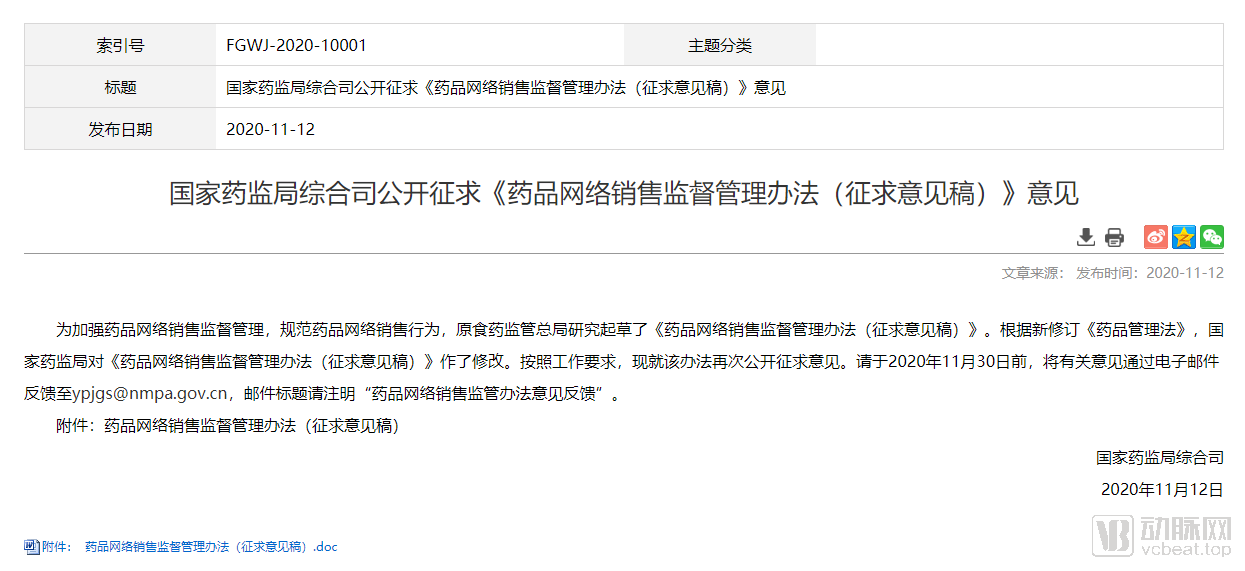
On November 12, the pharmaceutical e-commerce sector ushered in major benefits. The Comprehensive Department of the State Food and Drug Administration publicly solicited the “Measures for the Supervision and Administration of Online Drug Sales (Draft for Comment)”, officially indicating the direction for online prescription drugs and allowing online sales Prescription drugs and display prescription drug information.
In the past, vaccines, blood products, narcotic drugs, psychotropic drugs, toxic drugs for medical use, radioactive drugs, pharmaceutical precursor chemicals and other drugs subject to special management by the country are still not allowed to be sold online.
Online prescription drugs, breaking the ice step by step
Whether prescription drugs can be sold via the Internet has been a hot topic of discussion in recent years.
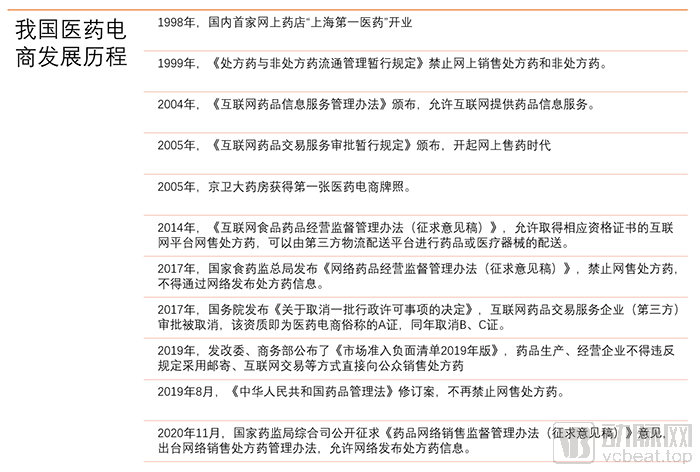
In the two editions of the “Measures for the Supervision and Administration of Online Drug Sales (Draft for Comment)” published in 2017 and 2018, it was clearly required that prescription drugs should not be sold online, and that websites selling drugs to individual consumers should not be published online Prescription drug information. The regulations have an important impact on the business of pharmaceutical e-commerce, especially online pharmacies. Online prescription drugs have encountered a one-size-fits-all regulatory dilemma, and the industry has been looking forward to the lifting of the ban on online prescription drugs.
On August 26, 2019, the newly revised “Pharmaceutical Administration Law of the People’s Republic of China” was promulgated. Drugs prohibited from selling onlineNo prescription drugs appeared on the list.
Some deputies to the National People’s Congress believe that online sales of prescription drugs should be strictly prohibited, and some deputies believe that online sales of prescription drugs cannot be one size fits all. In the subsequent press conference, the National People’s Congress listened to the opinions of all parties and adopted an inclusive and cautious attitude towards online sales of prescription drugs.
Yuan Jie, director of the Administrative Law Office of the Legal Work Committee of the Standing Committee of the National People’s Congress, talked about the issue of online prescription drugs. Director Yuan Jie said that online drug sales must comply with the relevant regulations on drug business, and online prescription drugs must adhere to the same online and offline standards and the principle of integrated supervision. The National People’s Congress authorized the State Council’s drug regulatory authority, and then worked with the State Council’s health authority to formulate specific measures.
Liu Pei, Director of the Policy and Regulations Department of the State Drug Administration, said that considering the general principles of the Drug Administration Law, the main body of online prescription drugs must first be an entity enterprise that has obtained a drug business license and has an offline license. Prescription drugs can only be sold online. Taking into account the particularity of online sales of prescription drugs, more stringent requirements are imposed on online sales of prescription drugs. For example, the drug sales network must be connected to the medical institution information system interconnection, information sharing. The main purpose is to ensure that the source of the prescription is true and to ensure the safety of the patient’s medication. In addition, in terms of distribution, it must meet the requirements of drug quality regulations.
Later, the revised version of the “Drug Administration Law of the People’s Republic of China” was officially implemented on December 1, 2019. This means that online sales of prescription drugs are not expressly prohibited, but specific regulatory measures have to wait for regulatory authorities to formulate them. In the drafting process.
However, “online shopping” has become one of the main ways of daily consumption for Chinese residents, and obtaining medicines through the Internet is favored because of its convenience and speed. On the second day after Double Eleven in 2020, the “Measures for the Supervision and Administration of Drug Online Sales (Draft for Comment)” revised in accordance with the requirements of the latest “Drug Administration Law” were posted on the National Medical Products Administration for public comments. The new Online drug supervision measures finally landed.
This is the rule for online prescription drug sales
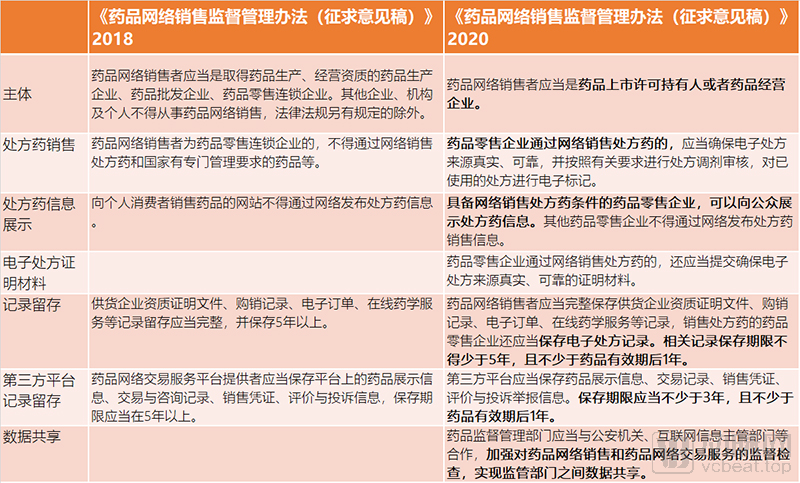
The draft for comments is divided into 6 chapters, including general provisions, drug online sales management, platform management, supervision and management, legal responsibilities, and supplementary provisions, with a total of 48 articles. The previous prohibitions related to the sale of prescription drugs have been changed in the new “Opinion Draft.”
First, on the premise of ensuring the authenticity and reliability of electronic prescription sources, online sales of prescription drugs are allowed;
It is written in Article 9 of the “Draft Opinions” that when pharmaceutical retail companies sell prescription drugs online, they should ensure that the source of electronic prescriptions is true and reliable, conduct prescription adjustment reviews in accordance with relevant requirements, and electronically mark used prescriptions . Pharmaceutical retail companies sell drugs through the Internet, and may not give prescription drugs and Class A non-prescription drugs to the public in the form of buying drugs as gifts, or buying commodities as gifts.
Previously, online sales of prescription drugs were banned because of regulatory considerations from a security perspective. Online drug supervision is different from offline supervision. It is difficult to distinguish between true and false prescriptions on the Internet, and the supervision responsibility is not clear. And because of the virtuality, concealment, and cross-regional characteristics of drug online sales, it is also difficult for the supervisory level to obtain evidence and punish. The media has previously exposed that some medical e-commerce platforms can also buy prescription drugs through audits with fake photos.
Therefore, the new version of the Opinion Draft requires that the authenticity of the prescription be reviewed to ensure the authenticity of the prescription.
Second, pharmaceutical retail companies that are qualified to sell prescription drugs online can display prescription drug information to the public. The drug information displayed by drug online sellers shall be true, accurate, legal and effective, and the drug approval number shall be indicated. Pharmaceutical retail companies that have the conditions to sell prescription drugs online should highlight the risk warning information such as “prescription drugs must be purchased and used under the guidance of a licensed pharmacist” when displaying prescription drug information to the public.
In the previous administrative measures, it was stipulated that websites selling drugs to individual consumers must not publish prescription drug information through the Internet. In a social environment that does not have the liberalization of online sales of prescription drugs, regulatory agencies must consider the overall situation to ensure safe medication. At that time, although there were many medicinesE-commerce networkThe station claims that it does not sell online, but the information of prescription drugs is displayed on the webpage, and there is a phenomenon of selling prescription drugs by scoring.
With the improvement of Internet supervision methods and methods, online prescription drugs can be gradually liberalized.
Sales of prescription drugs are released, and supervision needs to keep up
The draft of this consultation also made clear provisions on drug online sellers, online sales scope, online seller obligations, third-party platform management, drug distribution, and legal responsibilities.
The “Draft for Solicitation of Comments” clarifies the various requirements for drug online sales management. Online drug sellers should be drug marketing license holders (hereinafter referred to as holders) or drug business enterprises; drug online sales must not exceed business operations Method and scope of drug business.
Vaccines, blood products, narcotic drugs, psychotropic drugs, toxic drugs for medical use, radioactive drugs, pharmaceutical precursor chemicals, and other drugs subject to special administration by the state, shall not be sold online. Drug online sellers shall take measures such as stopping sales, recalling or recovering drugs that have quality problems or hidden safety hazards, and release corresponding information on the website or the main page of business activities in a timely manner.
In Article 16 of the “Draft Opinions”, there are also requirements for data record keeping. If drugs are sold to individuals, sales certificates shall be issued in accordance with regulations. The sales voucher can be issued in electronic form. Drug online sellers should keep complete records of supplier qualification certificates, purchase and sales records, electronic orders, online pharmaceutical services, etc., and drug retail companies selling prescription drugs should also keep electronic prescription records. The retention period of relevant records shall not be less than 5 years and not less than 1 year after the expiry date of the drug.
In terms of drug distribution, drug network sellers shall be responsible for the quality and safety of the drugs distributed, and ensure that the storage and transportation of drugs comply with the relevant regulations of the drug business quality management regulations. According to the quantity of drugs distributed, transportation distance, transportation time, temperature requirements, etc., select appropriate transportation means and temperature control methods to ensure that the transportation process meets the requirements and the distribution activities can be traced throughout.
The “Draft for Comments” pointed out that the third-party platform should review the qualifications of drug online sellers applying for entry; establish and implement a system to ensure drug quality and safety; establish a drug quality management agency to undertake drug quality management; establish a transaction a class=”project-link” data-id=”174343″ data-name=”易记” data-logo=”https://img.36krcdn.com/20200729/v2_d7628f4c80b9439cad507e8e2fa4a040_img_000″ data-refer-type=”2 “href=”https://36kr.com/projectDetails/174343” target=”_blank”>Easy to remember records preservation, complaint management and dispute resolution, and adverse drug reaction information collection systems; establish and implement a delivery quality management system.
The third-party platform shall keep drug display information, transaction records, sales vouchers, evaluation and complaint information. The shelf life should be no less than 3 years and no less than 1 year after the expiry date of the drug. The third-party platform shall adopt technical means such as electronic signatures, data backup, and failure recovery to ensure the authenticity, integrity and safety of materials, information and data, and provide convenience for the settled drug network sellers to save the above-mentioned data.
Policies are beneficial to the development of pharmaceutical e-commerce
As one of the important application scenarios and business formats of Internet medical care, the development of the pharmaceutical e-commerce field has been affected by policy uncertainty for a long time. Is the domestic policy for prescription drugs Can net sales are always in a swinging state.
Until the introduction of the new version of the Drug Administration Law last year, the uncertainty caused by policy risks was eliminated. According to Ai Kunwei’s data forecast, with the advancement of the division of medicines, the outflow of prescription drugs will be about 400-500 billion yuan, of which the retail market will be about 300 billion yuan. The opening of online prescription drugs has brought huge market space for pharmaceutical e-commerce, and the Internet + development of chain physical pharmacies has also eliminated more obstacles.
Dr. Yu Gang, the co-founder and executive chairman of 1Yao.com, said in an interview with Artery.com: “The Measures for the Supervision and Administration of Online Drug Sales (Draft for Comment)” once again publicly solicited opinions. The rigor and attention to this is a benefit to the Internet medical and health industry, which will help the long-term standardized development of the industry, and will promote enterprises to better provide convenient medical and health services to the people through new technologies.”
“Compared with the original draft, the State Food and Drug Administration has carefully listened to the feedback from industry companies represented by Yiyao.com, and has made great improvements in terms of prescription drug display and sales, which is in line with industry operations. In fact, it can meet the requirements of ensuring the safety of people’s medication, and it provides clear guidelines for the industry.”
In the prevention and control of the new crown pneumonia epidemic this year, Internet medical and health companies have played an important role. They have been recognized by society and the country. They have also greatly improved the public’s acceptance of Internet medical and health services, which has greatly promoted Policy forward. Dr. Yu Gang believes that the policy will be more open in the future.
With the implementation of the “Measures for the Supervision and Administration of Drug Online Sales (Draft for Comment)”, the sunny operation of online prescription drugs under supervision will benefit market regulation and sound development. Many policies, such as online sales and prescription drug outflow, separation of medicines, proportion of medicines, and medical insurance control fees, complement each other. Online and offline compete with each other and complement each other. I believe that pharmaceutical e-commerce companies will usher in the spring of development under the guidance of the new management measures.