The conditional listing application of Cansino Bio’s recombinant new coronavirus vaccine was accepted by the State Food and Drug Administration.
Cansino Bio issued an announcement in the afternoon on February 24 that it was a new recombinant type jointly developed with the Institute of Bioengineering, Academy of Military Medicine, Academy of Military Sciences. The conditional listing application for the coronavirus vaccine (adenovirus type 5 vector) (“Ad5-nCoV”, trade name Keweisha) was accepted by the State Food and Drug Administration. This means that Ad5-nCoV is only one step away from being approved in China, and the vaccine can be officially launched after the approval of the State Drug Administration.
In China, it will take about 6-8 months to complete the review items and issue the drug marketing authorization from the date when the new drug marketing application and related materials are delivered and the acceptance notice is obtained. However, in the event of a public health emergency, new drugs required for emergency treatment can be handled in accordance with special approval procedures. For the prevention and control of the epidemic, the State Food and Drug Administration urgently opened a green channel for emergency approval of medicines and medical devices this year to ensure the need for emergency prevention and control materials and the drugs and medical devices required for epidemic prevention. BGI’s new crown test kit took only 4 days to approve; Coxing Zhongwei’s new crown vaccine took only 2 days from the submission of the marketing registration application on February 3 to the approval. After the Sinopharm Group announced that the application for the new crown vaccine was accepted, Sinopharm-related stocks rose sharply in the afternoon, and Sinopharm (600511.SH) rose 6.90%.
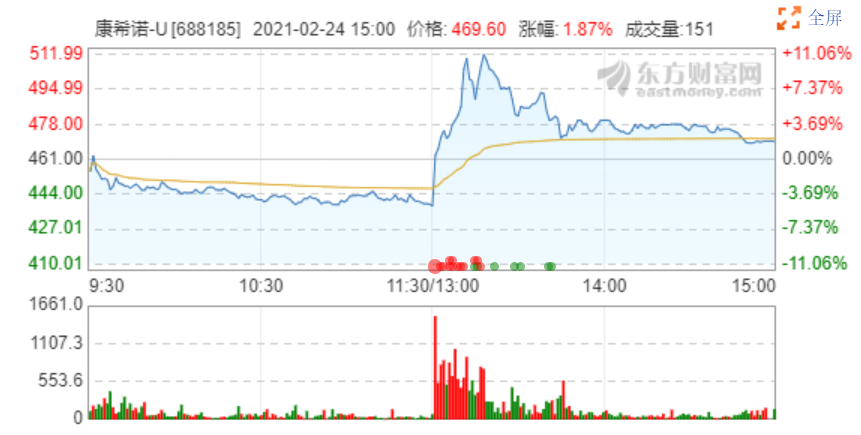
Cansino share price changes on February 24
The announcement stated that the vaccine has carried out a global multi-center phase III clinical study in five countries including Pakistan, Mexico, Russia, Chile and Argentina, and has completed the vaccination and interim data analysis of more than 40,000 subjects. The results of the analysis of the data during the phase III clinical trial showed that the overall protective efficacy of the vaccine against all symptoms was 65.28% after 28 days of single-shot vaccination; and the overall protective efficacy of the vaccine against all symptoms was 68.83% after 14 days of single-shot vaccination. The protective effects of the vaccine on severe illnesses were: 90.07% after 28 days of single-dose vaccination; 95.47% after 14 days of single-dose vaccination. Ad5-nCoV protection efficacy data results meet the relevant technical standards of the World Health Organization and the relevant standards in the “Guidelines for Clinical Evaluation of New Coronavirus Preventive Vaccines (Trial)” issued by the State Food and Drug Administration.
China’s first single-dose new crown vaccine
Ad5-nCoV is the world’s first new coronavirus vaccine that has entered the clinical research phase. The first and second clinical trials were launched in Wuhan on March 16 and April 12 last year.Bed test. With the recent approval of emergency use in Pakistan, Mexico and other countries, Ad5-nCoV has become the third domestically-made new crown vaccine to be vaccinated on a large scale. Xingzhongwei’s Kerlaifu.
Different from the inactivated virus vaccines of Sinopharm Group and Kexing Zhongwei, CanSino Bio’s Ad5-nCoV is an adenovirus vector vaccine. Traditional inactivated vaccines use inactivated viral microorganisms as antigens, and the immune effect produced by them is relatively short, and generally requires multiple vaccination. Adenovirus vector vaccine is to insert part of the gene sequence of the virus into the adenovirus to construct a fusion virus to stimulate the body’s immune response. Since the live virus can proliferate in the body and act on the body cells for a long time, it can induce strong Immunity. Although the adenovirus vector vaccine has high gene delivery efficiency and strong immunity to stimulate, compared with other vaccines, it has the disadvantages of potential safety risks of the virus vector and susceptibility to the inherent receptors of the host.
CanSino Bio’s Ad5-nCoV is the first new coronavirus vaccine in China that only requires a single injection. The vaccination procedure is simple, and it can protect more people under the same capacity; at the same time, the critical illness prevention rate of more than 90% can be greatly reduced Pressure from medical institutions at all levels. In addition, the new crown vaccine usually has higher requirements for transportation and storage conditions, while Ad5-nCoV can be stored at 2 to 8°C, which is convenient for transportation and storage.
With a pre-loss of 400 million yuan and a huge investment in research and development, can Cansino make a profitable “turnaround”
CanSino Bio was established in 2009 and will be listed on the Sci-tech Innovation Board on August 13, 2020, becoming the first “A+H” vaccine stock since the opening of the Sci-Tech Innovation Board.
In the 2020 annual performance forecast announcement issued by Cansino, the company expects that the net profit attributable to the owners of the parent company will be between -400 million yuan and -430 million yuan in 2020, compared with the same period last year. The loss increased by 240 million yuan to 270 million yuan, a year-on-year increase of 155.13% to 174.27%; it is estimated that the annual research and development expenses incurred in 2020 will be 440 million yuan to 470 million yuan, an increase of 290 million yuan to 320 million yuan compared with the same period last year , An increase of 189.96% to 209.73% year-on-year.
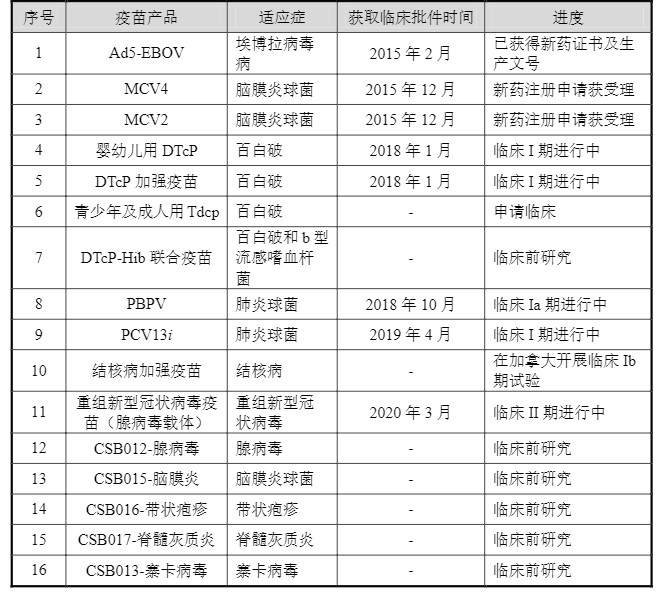
Consino Biotech is aimed at 16 innovative vaccines for the prevention of 13 indications including meningitis, Ebola virus disease, DPT, pneumonia, tuberculosis, recombinant new coronavirus vaccine (adenovirus vector), and herpes zoster. Product research and development, among which Ad5-EBOV, which is adapted to Ebola virus, is in the stage of obtaining a new drug certificate and a production number. This means that Cansino currently has no major products that can provide revenue on the market.
CanSino 16 innovative vaccine products
In March 2020, Ad5-nCoV took the lead to enter the clinical research phase, and the market value of Cansino Biologics has risen from the tens of billions to hundreds of billions of market value. It is also the R&D investment in the field of new crown vaccines that will further expand Cansino Bio’s loss in 2020. On February 18, Cansino-U-B (06185.HK) once expanded to 10%, and closed down 9.35% to close at HK$393.60 per share.
All this finally ushered in a turning point. After being approved for emergency use in Pakistan, Mexico and other countries, CanSino Bio’s Ad5-nCoV conditional listing application was accepted by the State Food and Drug Administration. If Ad5-nCoV is successfully listed, the advantages of its 1-shot vaccination procedure will gradually appear. Significantly reduce the cost paid by the society and the burden of medical institutions; for CanSino, this will also be the first step to usher in product commercialization and realize scale income.
According to Huaxi Securities, the new crown vaccine alone may generate up to 16.8 billion yuan in profits in the domestic market. In addition, China is providing vaccine assistance to 53 developing countries and has reached vaccine export agreements with 22 countries. From a global perspective, the market space for new crown vaccines for Chinese companies may reach more than 100 billion. As of February 21, the new crown virus vaccine developed by Sinopharm has used more than 43 million doses worldwide, and Indonesia has also reached a deal with China to order 125 million doses of Kexing vaccine.
The designed production capacity of Cansino’s Tianjin plant is 200 million doses per year. Based on a profit of 15 yuan per bottle, Cansino’s new crown vaccine profit will reach 3 billion yuan in 2021, and sales will reach 10 billion yuan. In addition, CanSino’s meningitis vaccine MCV4 and MCV2 will enter the commercialization stage in 2021. MCV4 has obvious product competitiveness but high vaccination costs (1350 yuan). The overall penetration rate of MCV4 is expected to be 30%, which will occupy 50%. % Market share, corresponding to the peak sales of 3.2 billion yuan. Therefore, it is believed that CanSino has an estimated loss of 400 million, and it is expected to realize a turnaround in 2021 with the commercialization of the new crown vaccine, MCV4, and MCV2; as the pneumonia vaccine, the DTP vaccine, and the tuberculosis intensive vaccine are gradually commercialized , Sales revenue is expected to reach the tens of billions level.
A once-in-a-century overtaking opportunity on a curve
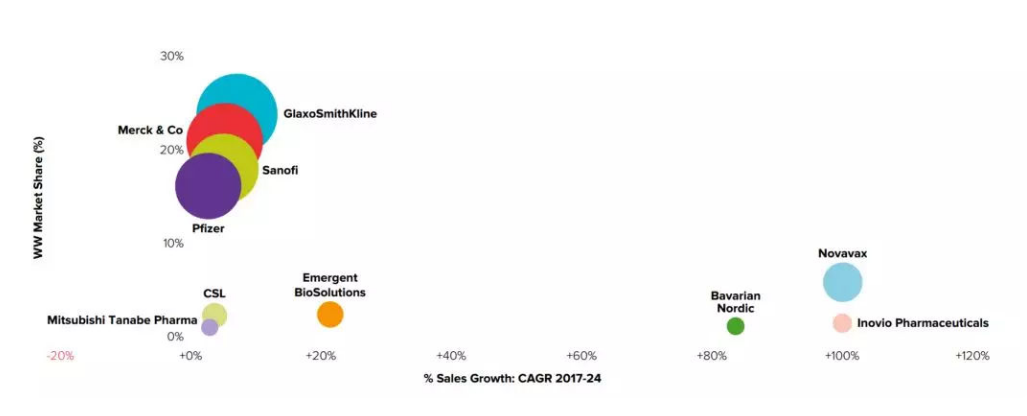
In the global vaccine market, European and American vaccination awareness is mature, and the market accounted for more than 60%; China is an emerging market for vaccines, with vaccine varieties and coverage rates at a relatively low level, and there are still many market gaps. The global competition landscape is also oligarchic competition, almost four companies headquartered in Europe and America, GlaxoSmithKline, Sanofi Pasteur, Merck and Pfizer.Pharmaceutical companies carve up the world, occupying 90% of the global market share (data from Gaotejia Investment).
The vaccine industry has high barriers to competition, and the late clinical stage usually requires high costs of hundreds of millions of dollars. Large pharmaceutical companies have strong financial resources, and their mature commercialization teams can also build global production capacity and accelerate market capture. On the contrary, cutting-edge companies can hardly tap opportunities from the four major vaccine giants.
Predict the global vaccine market share in 2024, data sources World Preview 2018, Outlook to 2024, pictures are from Gaotejia Investment’s official website
However, this competition for the new crown vaccine seems to give Chinese biotech companies such as Kangsino a once-in-a-lifetime opportunity to overtake on a curve. Among the world’s first eight vaccines to enter the clinic, China occupies four seats. They are: the recombinant new coronavirus (adenovirus vector) vaccine jointly developed by Academician Chen Wei of the Academy of Military Medicine of the Academy of Military Sciences and Cansino Bio, and Beijing Kexingzhong The new coronavirus inactivated vaccine developed by Wei Biotechnology Co., Ltd., and the new coronavirus inactivated vaccine developed by Wuhan Institute of Biological Products and Beijing Institute of Biological Products under Sinopharm Group.
There are three core difficulties in the development of innovative vaccines: “Long time, high investment, and low success rate.” Regarding the public health emergency of the new crown epidemic, local administrative agencies have given policy support to help shorten the vaccine development cycle; in addition, it is estimated that the research and development cost of the new crown vaccine is lower than other innovative drugs. It has brought a rare opportunity to overtake on a curve for various domestic cutting-edge companies.
In order to stop the spread of the epidemic as soon as possible, administrative agencies are trying to shorten the research and development cycle, and the time cost has been compressed to the extreme compared with normal drugs. This way of shortening the research and development cycle usually includes reducing the number of patients enrolled in clinical trials, sympathetic medication, accelerated approval, and conditional approval.
In March 2020, the State Administration for Market Regulation announced the “Administrative Measures for Drug Registration” and “Administrative Measures for Drug Production Supervision”, clarifying that vaccines and innovative vaccines urgently needed for disease prevention and control can apply for priority approval. The State Food and Drug Administration revealed in its answer to reporters’ questions on the “Four Strict” special actions on food and drug safety on February 19 that it has conditionally approved the listing of 2 new coronavirus vaccines in my country, and 5 technical routes totaling 16 have been approved for emergency. Clinical trials have been carried out on three vaccine varieties, and six vaccine varieties have started phase III clinical trials. Recently, Thailand, Argentina, the PhilippinesCountries such as Lubin have also approved the emergency use of the new crown vaccine of China Kexing and Sinopharm Group.
CEPI (Alliance for Epidemic Prevention Innovation) is funding 8 leading new crown vaccine candidates worldwide to enter clinical trials, and the goal is to have at least 3 candidate vaccines submitted for marketing by 2021. According to its survey and calculation, in order to achieve this goal, it needs to invest about 2 billion US dollars. According to Xinkangjie’s calculations, the research and development costs are between 379.2 million US dollars and 675 million US dollars according to the success rate. Looking at the H1N1/H5N1 influenza vaccine research and development costs horizontally, the estimated result is 372.5 million US dollars, which is close to the lowest cost of new crown vaccine research and development. This amount of capital investment is still relatively low compared to other innovative drugs that cost 1 to 2 billion US dollars.
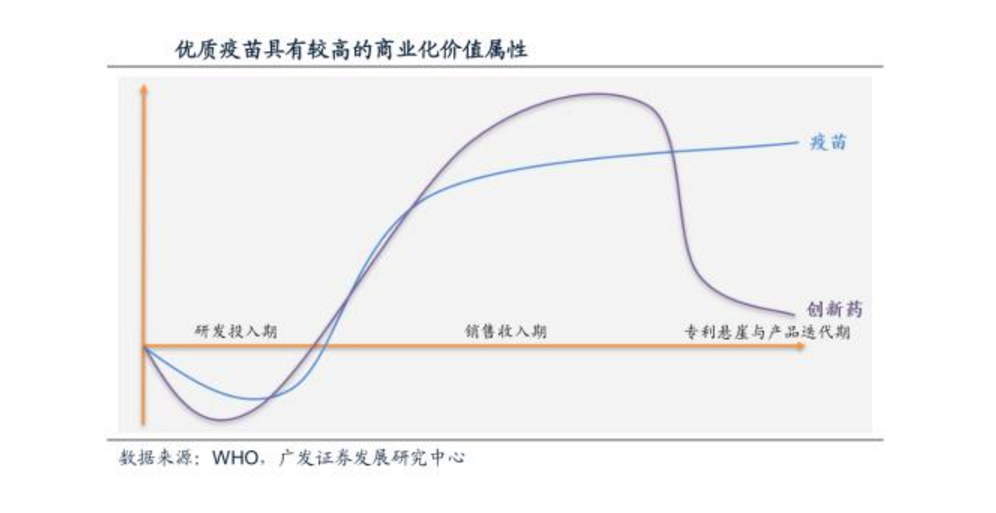
Data source: WHO, GF Securities Development Research Center