“In the beginning of the epidemic, it cost 200 yuan to do a nucleic acid at your own expense, and each time you finished it, you kept the invoice to see if it could be reimbursed. Later, the price continued to drop, first more than 100, and then to double digits, and you can do it once for 30 to 40 yuan. , and will not be particularly concerned about reimbursement.” This is an ordinary citizen’s intuitive impression of the price changes of nucleic acid testing since the new crown epidemic in early 2020.
The price of nucleic acid testing continues to drop. Recently, Hebei, Henan, Beijing and other places have lowered the price of nucleic acid testing again. The lowest price of mixed testing has reached 3.4 yuan. In the centralized procurement of local organizations, the single price of nucleic acid is as low as 3.2 yuan.
Not only nucleic acid, but also the unit price of the new crown antigen test, which constantly refreshes the low price, some companies quoted the lowest price of 3.91 yuan in centralized procurement. In contrast, when it was approved in China in mid-March this year, its retail unit price was around 30 yuan, while the current price for a single serving on e-commerce platforms is around 10 yuan.
Nucleic acid detection is the gold standard for judging new coronavirus infection, and antigen detection is an important supplementary method. From the perspective of the general public, the continuous decline in prices is a good thing; for companies that have won bids, they need to think about how much room there is for business growth brought about by the new crown testing after the price continues to decline, and with the ups and downs of the epidemic, what will companies rely on? Get great development.
The lowest price of new crown nucleic acid is 3.2 yuan
Nucleic acid testing has been widely used in the early stage of the domestic new crown epidemic, and the price reduction has already started in April 2020. At that time, the Hubei Provincial Pharmaceutical Equipment Centralized Procurement Service Platform released the “Central Procurement Documents for Novel Coronavirus-Related Testing Reagents in Hubei Province”, and Hubei Province officially launched the centralized procurement of novel coronavirus-related testing reagents, which was finally compared with the average purchase price of Hubei Province in the previous month. A drop of 81%.
It is expected that the price will drop, but it has since dropped to single digits, which has surprised many people in the industry.
On April 29, the Guangdong Alliance for the procurement of new crown testing reagents and related consumables, organized by the Guangdong Provincial Drug Exchange, announced the results of the selection. The regions include Shanxi, Inner Mongolia, Liaoning, Heilongjiang, Zhejiang, Jiangxi, Hunan, Guangdong, Guangxi, Hainan, Chongqing, Guizhou, Yunnan, Shaanxi, Gansu, Qinghai, Ningxia, Xinjiang, Xinjiang Construction Corps and other 19 provinces (cities, districts) ), the minimum quotation for nucleic acid is 3.2 yuan, and the quotation unit is Fosun Diagnostic Technology (Shanghai) Co., Ltd.The company’s general nucleic acid reagents and kits. 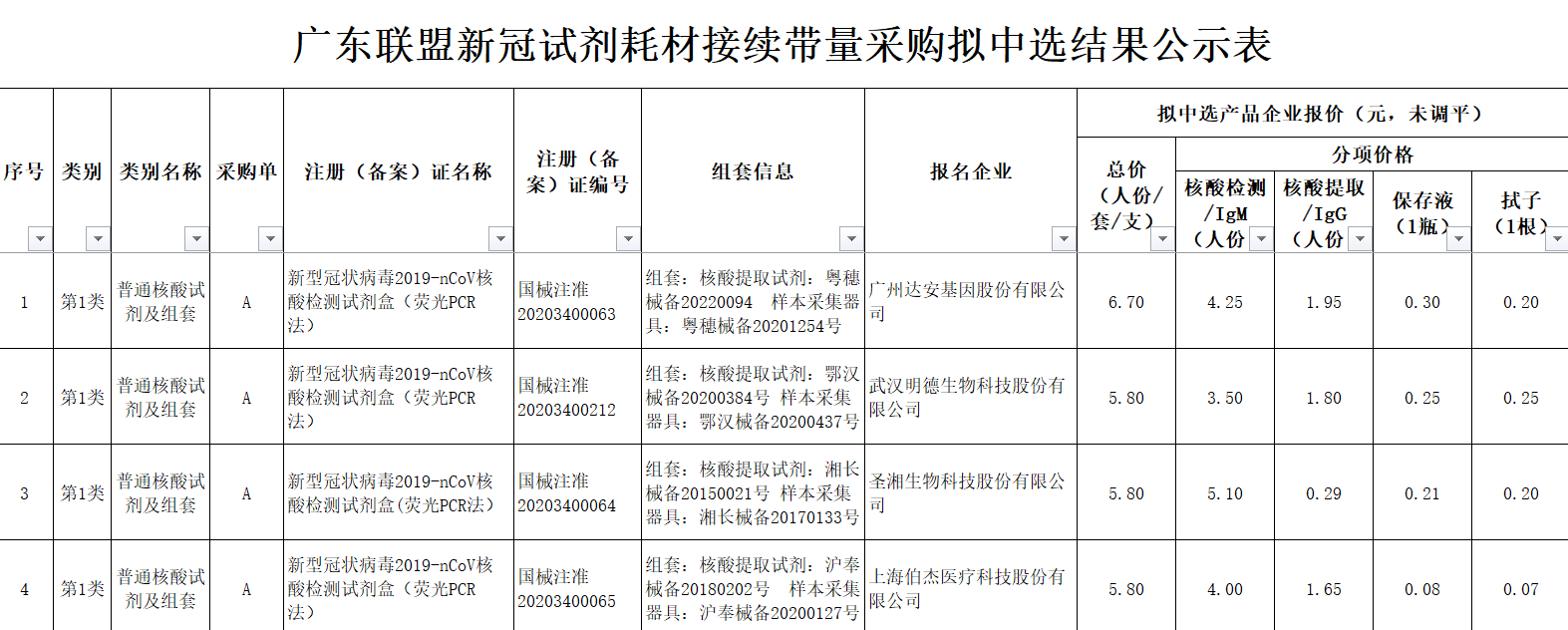 The above-mentioned Hubei pilot and the latest 19 provinces District centralized procurement is the purchase price of the enterprise’s government orders. A number of provinces and regions have issued price reduction notices, which directly affect the price charged for nucleic acid production by ordinary people.
The above-mentioned Hubei pilot and the latest 19 provinces District centralized procurement is the purchase price of the enterprise’s government orders. A number of provinces and regions have issued price reduction notices, which directly affect the price charged for nucleic acid production by ordinary people.
On May 5, Hebei issued the “Notice on Adjusting the Price of New Coronavirus Nucleic Acid Testing Items”. The maximum price has been reduced from 28 yuan per person to 19.7 yuan, and the maximum price of mixed testing has been reduced from 6 yuan to 3.4 yuan per person.  At the end of April, Henan issued the “Notice on the Price of Coronavirus Nucleic Acid Testing Items”, the price of the new crown nucleic acid detection (specimen apheresis) project has been reduced from 30 yuan/person to 18 yuan/person (excluding nucleic acid detection amplification reagents), and the new crown nucleic acid detection (specimen mixed collection) project The price is reduced from 10 yuan/person to 8 yuan/person (including related reagents and consumables).
At the end of April, Henan issued the “Notice on the Price of Coronavirus Nucleic Acid Testing Items”, the price of the new crown nucleic acid detection (specimen apheresis) project has been reduced from 30 yuan/person to 18 yuan/person (excluding nucleic acid detection amplification reagents), and the new crown nucleic acid detection (specimen mixed collection) project The price is reduced from 10 yuan/person to 8 yuan/person (including related reagents and consumables).
At the beginning of April, Beijing made the 7th dynamic adjustment of the new crown nucleic acid detection project. Since April 9, the price of a single sample of new crown nucleic acid detection in Beijing public medical institutions will be increased by each 35 yuan per sample was reduced to 24.9 yuan, and the mixed detection price was simultaneously reduced from 8 yuan per sample to 5.9 yuan.
Antigen test price dropped to 3.91 yuan within two months
It took one or two years for the price of nucleic acid to drop, while the drop in antigen test is more rapid.
On March 11, the official website of the National Health and Health Commission issued the “Notice on Printing and Distributing the New Coronavirus Antigen Detection Application Plan (Trial)”, indicating that on the basis of nucleic acid detection, Added antigen testing as a supplement, and organized the formulation of the “New Coronavirus Antigen Testing Application Program (Trial)”, which is the first time to clarify the relevant policies for community residents to self-test the new crown.
Since then, new crown self-test products have been approved one after another. As of April 27, the State Food and Drug Administration has approved 30 new crown virusAntigen detection reagent products. At the same time that the product was approved, the policy of reducing the price of antigen testing was also introduced soon.
On March 25, the Office of the National Medical Security Administration issued the “Notice on Strengthening the Price Management of Novel Coronavirus Antigen Testing”, clarifying that each provincial medical security department has The total fee for testing “price items + testing reagents” shall be capped. At this stage, the capping standard cannot be higher than 15 yuan per person. If conditions are met, the capping standard can be lowered to a more reasonable level. The notice also proposes that the price of new crown antigen testing items in public medical institutions should not be higher than 5 yuan per person.
When the joy of the company’s product approval has not gone far, centralized procurement has entered the field of antigen detection.
On March 30, Tianjin Pharmaceutical Purchasing Center and Hebei Provincial Medical Drug and Equipment Centralized Purchasing Center announced the purchase price of the third batch of Tianjin-Hebei novel coronavirus antigen detection reagents. , the novel coronavirus (2019-nCoV) antigen detection kit (colloidal gold method) of Lepu Diagnostics 9 yuan/person, the novel coronavirus (2019-nCoV) antigen detection kit of Guangzhou Wanfu Biotechnology Co., Ltd. ( Colloidal gold method) 7.9 yuan / person. 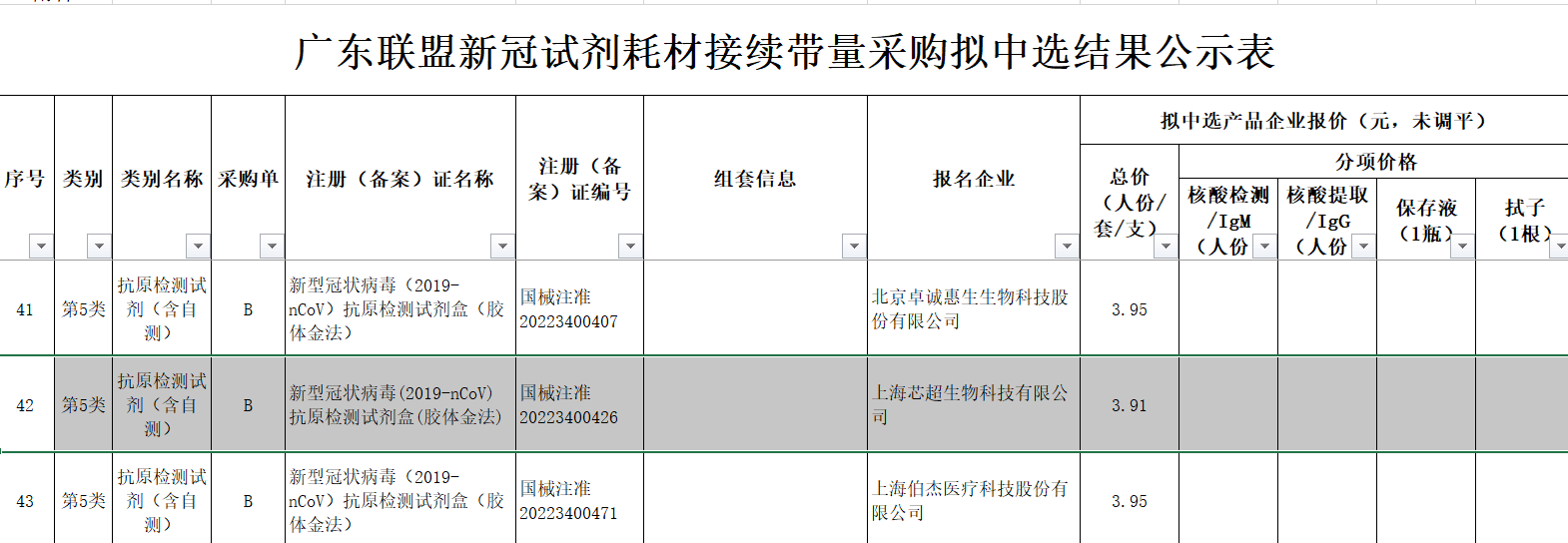 Descent from double digits to single digits , the price reduction of antigen testing has not stopped. According to the results of the Guangdong Alliance’s new crown detection reagents and related consumables procurement continuation work announced by the Guangdong Provincial Drug Trading Center, the antigen detection reagents (including self-tests) of Shanghai Xinchao Biotechnology Co., Ltd. reported the lowest price of 3.91 yuan.
Descent from double digits to single digits , the price reduction of antigen testing has not stopped. According to the results of the Guangdong Alliance’s new crown detection reagents and related consumables procurement continuation work announced by the Guangdong Provincial Drug Trading Center, the antigen detection reagents (including self-tests) of Shanghai Xinchao Biotechnology Co., Ltd. reported the lowest price of 3.91 yuan.
How much will the price cut affect related companies?
Under the new crown epidemic, nucleic acid detection products and antigen detection products have become a powerful driving force for the performance of many companies, which are called “laying earning” in the industry.
Taking Shengxiang Bio (688289), the “first anti-epidemic stock” as an example, it will achieve revenue of 4.763 billion yuan in 2020, a year-on-year increase of 1204%; The profit was 2.617 billion yuan, a year-on-year increase of 6528%. The 2020 annual report shows that these products serve the frontline of epidemic prevention and control in nearly 160 countries and regions around the world, and help thousands of laboratories at home and abroad to develop nucleic acid detection capabilities from scratch.multiplied to dozens of times. From the perspective of gross profit margin, the gross profit margin of diagnostic preparations where nucleic acid testing is located is as high as 87.41%.
However, the high growth of Shengxiang Biotechnology did not continue. 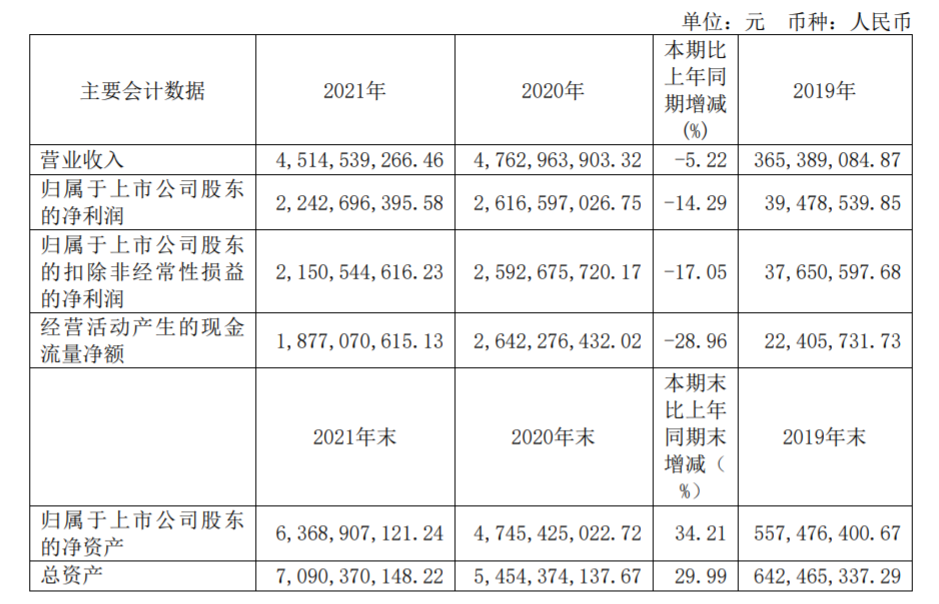
Main financial data of Shengxiang Bio in 2021
The 2021 annual report shows that the operating income of Shengxiang Bio is 4.515 billion yuan, a year-on-year decrease of 5.22%. The net profit attributable to shareholders of listed companies was 2.243 billion yuan, a year-on-year decrease of 14.29%. In this regard, Shengxiang Bio explained that due to factors such as the centralized procurement of new coronavirus detection reagents in China and the general price reduction of new crown-related products, the company’s operating income and net profit during the reporting period decreased slightly year-on-year. The gross profit margin of diagnostic reagent products also fell by 5% compared to 2020.
The benefits of antigen testing are reflected in the first quarterly report of this year. For example, the operating income of Wanfu Bio (300482) in the first quarter was 2.625 billion yuan, a year-on-year increase of 276.87%; The profit was 904 million yuan, a year-on-year increase of 481.32%; the non-net profit attributable to the parent was 899 million yuan, a year-on-year increase of 535.5%.
Wanfu Bio did not mention its future performance expectations in its quarterly report, but the competition in the antigen field and the impact of price, Wanfu Bio’s overseas market in 2021 tasted.
In the 2021 annual report, Wanfu Bio mentioned that in the second half of 2021, due to the increase in the number of companies that have obtained the EU CE new crown antigen test product registration access, the market for new crown antigen products will increase. With the increase in supply, the quotations of new crown antigen detection products in the European open market have a gradual downward trend, resulting in a decline in the gross profit margin of the company’s new crown antigen business compared with the same period in 2020.
However, the positive impact of nucleic acid testing business on corporate performance has not completely disappeared. According to a research report of Founder Securities on April 25, nucleic acid testing has become normalized under the “dynamic clearing” policy, and the demand has increased significantly. In the two years since the epidemic, the price reduction of nucleic acid detection reagents has continued to slow down. The market pattern is highly concentrated to leading companies, and the scale effect has led to outstanding profitability.
What to rely on after the new crown test?
“The new crown test is only a phased thing. You can’t rely on the new crown test for a lifetime. In the end, you must rely on the main business to survive in the industry.” A third-party testing agency In an interview with reporters, the person in charge of the company said that the new crown testing business has provided relevant companies with opportunities for skyrocketing, and has also helped companies develop markets and brands, but long-term development still depends on their main business.
Wanfu Biotech mentioned in its 2021 annual report that the supply of new crown products is guaranteed, conventional products maintain the basics, and new products promote growth. Focusing on the fields of acute and critical illness, common diseases, and frequently-occurring diseases, the company continuously conducts in-depth research on methods and methods such as organizational optimization, incentive design, and whole-chain management to promote quality improvement, innovate and continuously enhance product power.
Daan Gene (002030) is also a testing company that has grown up relying on new crown nucleic acid testing. The company once said on the interactive platform that it will continue to focus on its main business and clinical practice in the future. Diagnostic technologies and products.
Internationalization is also an important development direction for various testing companies.
Shengxiang Bio mentioned in its 2021 annual report that on the basis of the previous “7+2” international regional layout, the company has further formulated the “5+10” overseas The strategy will focus on the five major regions of Europe, South Asia and Oceania, North Asia CIS, Middle East and Africa, and the Americas, ten countries including France, the United Kingdom, and the Philippines, and further focus on key countries and key markets for in-depth cultivation.