Both commercialization and technical input and output need to be paid attention to
Editor’s note: This article is from the WeChat public account “deepatom” (ID: deepatom), author: deep dive atomer.
The annual CMEF Spring Fair is over, but its long-term impact on the medical industry has not faded. During the CMEF, gene technology company Genetron Health announced a strategic cooperation with Siemens Medical. The two parties will join hands in large-scale applications in the Chinese hospital market. This cooperation seems to lead the development direction of the genetic testing industry and has the opportunity to improve the quality of life of patients . In addition, Genetron Health also targeted the out-of-hospital market. Unlike the smooth sailing in the in-hospital market, its C-end market is currently lacklustre in the face of insufficient qualifications, high prices and insufficient brand power.
The term “pan-genesis” is derived from Darwin’s “pan-genesis theory”. It is the true source of the term “gene” and is the most primitive definition of genetic material by mankind. The name of the company directly explains the foundation of the business and empowers medical care through genetic technology. Genetron Health Sciences is also one of the first genetic technology companies in China to enter the field of cancer precision medicine. Based on the basic business of in vitro diagnostic (IVD) technology and clinical laboratory self-built project (LDT), it has derived cancer diagnostic monitoring, early cancer screening and drugs. Research and development of three major businesses.
△Darwin
In the future blueprint of Genetron Health Care, it covers cutting-edge technology and early cancer screening services, but the current R&D capabilities of Genetron Health are not outstanding, and the number of invention patents is not dominant compared with friends, and the technology is relatively weak. Ability may be a major hidden danger in its future development.
01
Under the epidemic situation, the business growth rate is insufficient and the wolfishness is reduced
In 2015, Wang Sizhen and Yan Hai co-founded Genetron Health, which uses genetic testing technology to achieve accurate cancer diagnosis. Unlike many companies that have disappeared in the dust of history, Genetron Health, which successfully went public in the United States in 2020, is undoubtedly the victor in this war.
In 2013, Hollywood superstar Angelina Jolie had her breast removed for self-protection after sequencing, and used a “radical” way to popularize gene sequencing. By 2016, in large hospitals in first-tier cities, most doctors are deeply involved in genetic testing, whether in scientific research or in the clinical field, and there is no doubt about the utility of this technology. This means that since 2016, genetic testing products have already had the basis for commercialization, and the establishment of Genetron Health has enjoyed the benefits of time.
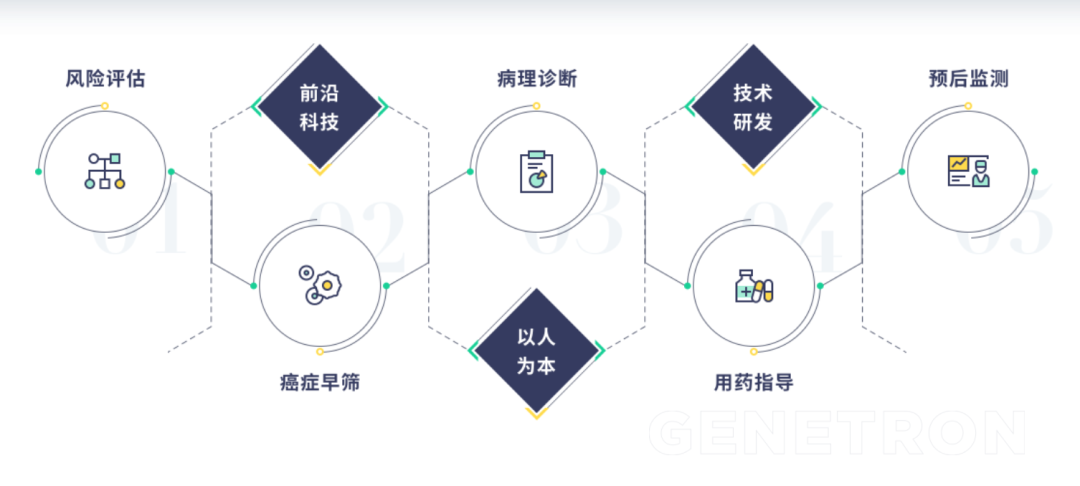
Most start-up companies will concentrate their energy on a certain product, accumulate enough advantages and then expand horizontally. However, Genetron Health is not the case. Genetron Health’s business basically covers cutting-edge technology and early cancer screening, and then subdivided into other clinical channels.
△Generic Biotech’s business direction
Business level in vitro diagnostic (IVD) technology and clinical laboratory self-built projects (LDT) are the basic businesses of Genetron Health, and it has derived three main businesses: cancer diagnostic monitoring, early cancer screening, and drug development services. Covers the full cycle of cancer from early screening of high-risk populations to patient diagnosis and treatment recommendations, as well as prognostic monitoring and management. The main products include liver cancer detection product HCCscreen™, pan-cancer molecular diagnostic solution OncoPanScan™, and new crown detection kits, etc. .
Developing in so many directions at the same time may be because Wang Sizhen, who achieved financial freedom in his 30s, has an ambition to promote the development of genetic technology; or Wang Sizhen, who has successful experience, hopes to achieve a greater cause. From the perspective of deep dive atom, the multi-service development model has made Panshengzi today and may also restrict its future development.
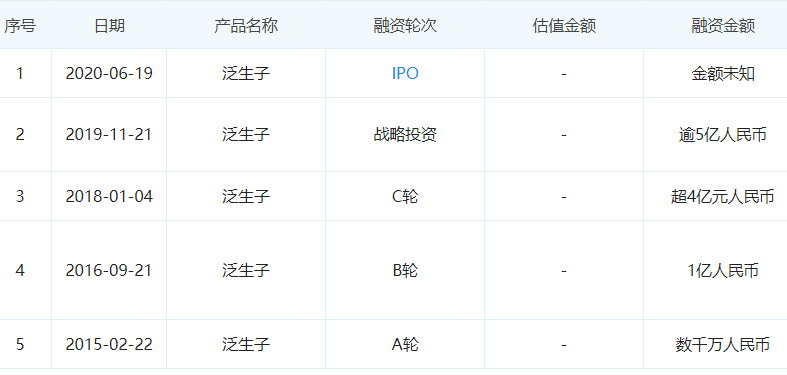
With the rise of precision medicine, genetic technology that can accurately detect diseases quickly entered the capital market. Under the leadership of Wang Sizhen, whose entrepreneurial products were successfully acquired before, Genetron Biotech is undoubtedly the darling of the capital market in such an environment. Within four years of its establishment, it completed 4 rounds of financing of more than 1 billion yuan. In June 2020, it successfully landed on the Nasdaq in the United States and completed the initial public offering, with a total fundraising of US$256 million.
△Finance Development History of Panshengzi
In 2020, genetic testing services have been popularized all over the world under the epidemic, but Genetron Health has not ushered in its own outlet. The total revenue for the whole year of 2020 is 424.5 million yuan, only an increase of 100 million yuan over 2019. The net loss for the current period in 2020 is 3.1 billion yuan, which is a significant increase from the 676 million yuan in 2019.
Before the listing, Genetron Health’s revenue was dominated by drug R&D services, which once accounted for 67.03%, and its business covered central nervous system cancer, lung cancer, liver cancer, colon cancer, breast cancer, etc. Today, Genetron Health’s HCCscreen™, an early screening test product for hepatocellular carcinoma, has been commercialized. In 2020, the revenue of LDT testing products HCCscreen™ and IVD products (including Genetron S5 instrument and lung cancer 8-gene kit) will increase significantly, with a gross profit margin of 61.3% in 2020 and 44.8% in the same period in 2019.
In 2020, the total number of LDT diagnostic tests was approximately 21,900, and the number of LDT tests decreased slightly by 4.1% year-on-year. However, the revenue from LDT services in 2020 was RMB 291.7 million, which was RMB 234.6 million from the same period in 2019. , An increase of 24.4% year-on-year. The trend of genetic testing has increased the value of Genetron Biotech’s products. However, with the downward price adjustment of the entire industry, how long can Genetron Biotech’s revenue growth last?
While the industry is swiftly racing and enclosing the land, Genetron Health has slowed down its pace. Its sales expenses have dropped from 254 million yuan in 2019 to 247 million yuan, and sales expenses accounted for 78.4% in 2019. Reduced to 58.2%. Entering 2021, Genetron Health and Chia Tai Tianqing will jointly promote the HCCscreen™ product to enter the hospital channel. In May, it also cooperated with Siemens Healthcare. According to Frost & Sullivan (Frost & Sullivan) data, the market potential of early liver cancer screening in China is expected to reach US$7.2 billion in 2023. When there is an urgent need for a race to an enclosure, it may not be wise to take the initiative to slow down one’s progress and place expectations on other companies.
02
NoneAccording to risk-based early screening outside the hospital, technical foundation needs to be improved
With the maturity of genetic testing technology, the cancer cycle is roughly divided into four stages: prediction, early screening, diagnosis, treatment and monitoring. Genetron Health is currently mainly used in cancer diagnosis and tumor precision treatment, tumor liquid biopsy, etc. to launch research and development and applications.
Liver cancer has become a happy nightmare for tens of thousands of families. Among all cancers, liver cancer mortality ranks second, with a 5-year survival rate of only 12.1%. Globally, there are 854,000 new cases of primary liver cancer each year, while China has 466,000 cases, accounting for 54.6% of the global number of liver cancer patients. In early 2019, Genetron Health has published China’s first prospective study on early screening for liver cancer, which has been published in the Proceedings of the National Academy of Sciences.
Subsequently, the HCCscreen ™ product was launched, which can achieve precise detection of common liver cancer mutations such as cfDNA point mutations, indel mutations, and HBV virus integration. Of course, this also opened the way for Pan Health to screen and greatly increased Pan Health’s Imagination. However, in the early cancer screening, there is a difference between the in-hospital market and the out-of-hospital market. Different scenarios have different requirements for products, and the corresponding operating environments are also different.
In the hospital market, genetic testing products are more convenient to operate. There are professional medical staff to take samples, and the whole process is safe and controllable. Among them, medical examination institutions are the most typical application market. Although Genetron Health Medical has already launched in-depth cooperation with iKang Guobin, the most difficult aspect for Genetron Health is in channel expansion. The cooperation with Chia Tai Tianqing and Siemens Medical can help Genetron Biotech’s channel expansion, but decentralizing channels also means reducing their motivation to proactively attack. How to find a balance in this kind of cooperation is very important .
In the big health track, compared with medical institutions with limited capacity, there is obviously a broader application market outside the hospital, which is theoretically more suitable for genetic testing products. Especially after the universal education of throat swabs and the new crown vaccine, the consumer’s acceptance of genetic products has been much higher than in the past. However, home screening poses the challenge of sampling. Genetron Health’s genetic testing products based on blood samples are highly dependent on medical staff. Self-sampling of blood may not only risk contamination of the samples, but also risk infection and other uncertainties for users.
As a medical product, if you want to enter the consumer market “innocently”, you must have corresponding qualifications. It is reported that Genetron Health plans to start the NMPA-registered clinical trial of HCCscreen™ in the second quarter of this year, but related products have already been sold on the consumer side. In Genetron Health’s flagship store on JD.com, we found that it has put a number of genetic testing products on the shelves, completely ignoring industry guidelines and user safety risks.
△Jingdong Store
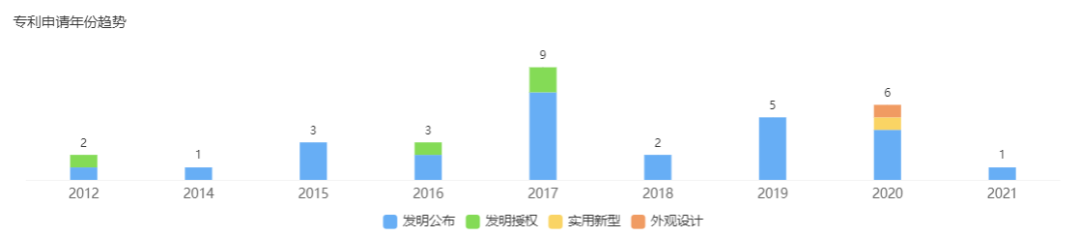
Under the grand blueprint of Genetron Health Care, it must be supported by hard enough technology. However, the technical capabilities of Genetron Health still need to be improved. At present, there are 32 patent applications recorded by Genetron Health, of which 4 invention patents have been rejected, 8 authorized patents and 5 public patents. In addition, most of them are still in the substantive examination stage. There is still a certain gap between the number of patents that can be used directly compared with Berry Gene and Burning Rock Medicine. Perhaps Genetron Health has discovered its own R&D capabilities. R&D expenses in 2020 will increase by 62.5% year-on-year, from 91.7 million yuan in 2019 to 149 million yuan, but it is not easy to catch up with its opponents in a short period of time.
△Patent application year
As an important direction of precision medicine, genetic technology has become an important part of medical technology and has begun to enter all aspects of medical treatment. In the future, genetic testing will be a basic technology in medical care. Behind every test is the future of a person or even a family.
Let’s look at a genetic technology company. In addition to its commercialization, it also needs to pay attention to its technical input and output. The genetic testing technology is very complicated, and it cannot be overcome in three or two years. It is hoped that Genetron Health, who is aware of its lack of technology, can polish up a better product.