The application of multi-omics technology in the field of tumor liquid biopsy is becoming more and more mature.
It is learned that Shenzhen Tailai Biotechnology Co., Ltd. (hereinafter referred to as “Talai Bio”) has completed a round of financing of nearly 100 million yuan, led by CDH Investment , Taiyu Investment, for the follow-up investment of Capital, Probe Capital acted as the exclusive financial advisor for this round of financing.
It is reported that this round of financing will be used for tumor liquid biopsy screening and diagnostic product research and development, market expansion, medical laboratory expansion, GMP production base construction and the second category, Clinical application for three types of IVD kits.
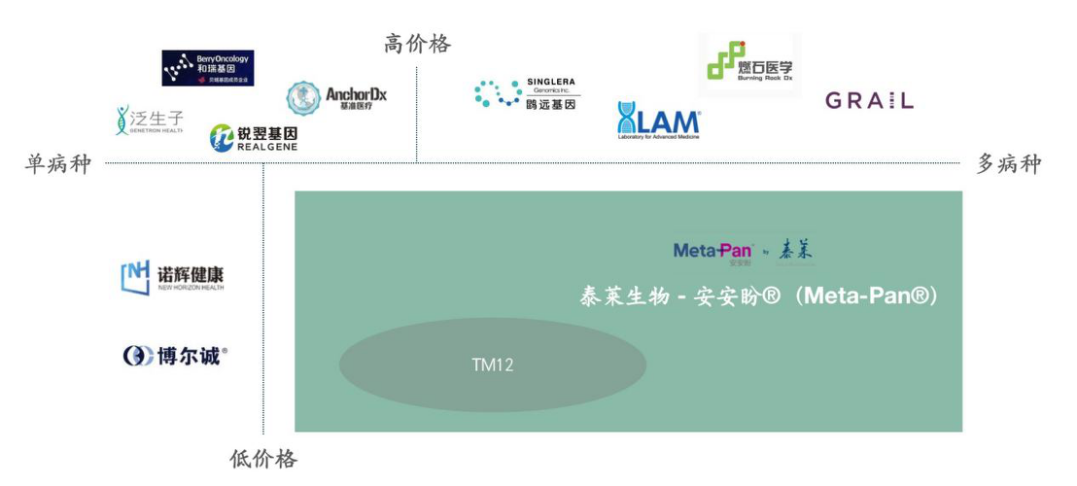
Taylor Biotechnology is a high-tech biotechnology company with detailed reports on it. It mainly provides innovative liquid biopsy products and services for major diseases. It currently focuses on the field of oncology. It has a number of multi-omics-based Tumor screening and diagnostic product pipeline, including high-throughput pan-tumor screening products, and clinical auxiliary differential diagnosis products for multiple tumor single diseases.
One of the high-throughput pan-tumor screening products——Meta-Pan® can treat 14 kinds of tumors with high incidence in China, such as lung cancer, stomach cancer, liver cancer, colon cancer, breast cancer, and prostate cancer. (12 species for men and 13 species for women) one-to-one early risk warning.
It is reported that this product uses the matrix-assisted laser analysis and ionization time-of-flight mass spectrometer (MALDI-TOF-MS) and 3D nano-material matrix independently developed by Taylor Biosciences, and Developed through the cross application of multi-omics and clinical medicine, artificial intelligence algorithms, materials science, and engineering.
Photo courtesy of the team
At present, Ananpan® has established a third-party medical laboratory in Chengdu and other places to provide medical examination institutions, insurance institutions, local hospitals, local public institutions and individual users in many provinces across the country with a terminal charge of 1,000 yuan. generalSieve service. As of December 2020, dozens of physical examination centers within Taylor’s United Nations have completed pan-tumor screening tests for tens of thousands of real-world physical examination populations.
According to Liu Yaokun, CEO of Taylor Biologics, for gastrointestinal endoscopy, CT, MRI and other commonly used clinical tumor diagnosis “gold standard” methods, the compliance of the Chinese grassroots is generally not high, the market needs innovative “non-invasive” General screening-level pan-cancer screening products”; In addition, with the acceleration of life in recent years, the incidence of tumors is showing a younger trend, and those under 40 years old and no genetic history have not been clinically recognized as “recommended gold standards” Consumers of healthy people who are the subject of testing also need “convenient and traceable pan-cancer screening products.” AnAnpan® was born based on this goal.
Photo courtesy of the team
It is reported that based on the high-throughput pan-tumor screening technology used by Ananpan®, Taylor Biosciences has cooperated with dozens of top three hospitals and hospitals in Zhongshan Hospital of Fudan University. National-level scientific research institutions have cooperated to complete the research and verification of more than 20,000 clinical samples. Among them, the results based on liver cancer, bowel cancer, pancreatic cancer, ovarian cancer, lung cancer research cohort have been published in ESMO, CSCO, ASCO, SGO, WCLC and other international academic Announced at the conference.
In terms of single-disease tumor clinical diagnostic products, Tailai Bio’s product development is mainly based on the combination of epigenetics, metabolomics, proteomics, genomics, imagingomics and other omics. Tumor assisted diagnosis, differential diagnosis, medication guidance for complex diseases, cancer recurrence monitoring and other fields have clinical needs. Currently, several product pipelines have been established in lung cancer, pancreatic cancer, liver cancer, gastric cancer, and glioma.
It is reported that the team has cooperated with a number of three parties, including West China Hospital Affiliated to Sichuan University, Fujian Union Hospital, Mengchao Hepatobiliary Hospital, and Cancer Hospital of the Chinese Academy of Medical Sciences.Hospital A and domestic and foreign tumor pharmaceutical companies have cooperated, and preliminary results have been published in journals such as “Nature Medicine”, “Cell Research”, “Clinical Epigenetics”, and “ESMO Open”. In addition, several clinical pipelines of Taylor Biologics are about to carry out a national multi-center clinical large-scale sample cohort prospective study next year.
In terms of the team, Stanley Lau, CEO of Taylor Biologics, has 40 years of experience in the medical, pharmaceutical and health industries. He has served in well-known multinational companies such as Merck, Schering-Plough, and Baxter. Senior management positions such as general manager and president of the Greater China region of the top 500 pharmaceutical companies.
Extended reading
First release | Focusing on multi-omics liquid biopsy, “Taylor Bio” received tens of millions of Pre-A round financing