Since the outbreak of the new crown epidemic, it has not only posed a global threat to public health, but has also placed a heavy burden on the medical system and the global economy. In response to this pandemic, countries around the world have made unprecedented efforts, including the development of antiviral drugs and vaccination.
Recently, academician Gao Fu, director of the Chinese Center for Disease Control and Prevention, and director of the Key Laboratory of Pathogenic Microbiology and Immunology of the Chinese Academy of Sciences, and others issued a reminder that when formulating epidemic prevention and control strategies, we still There are many factors to be aware of. This viewpoint article was published in China CDC Weekly, entitled “COVID-19 Expands Its Territories from Humans to Animals”.  Gao Fu et al. pointed out that in SARS-CoV During the spread of -2, several noteworthy variant strains (VOC) were gradually discovered. Current research has also proved that some of these variant strains have higher transmission capacity and are more resistant to the recovery period and the vaccine serum. And its role, posing a global threat to public health.
Gao Fu et al. pointed out that in SARS-CoV During the spread of -2, several noteworthy variant strains (VOC) were gradually discovered. Current research has also proved that some of these variant strains have higher transmission capacity and are more resistant to the recovery period and the vaccine serum. And its role, posing a global threat to public health.
They believe that the arms race between the evolution of SARS-CoV-2 and human response strategies will continue for some time.
The origin of SARS-CoV-2 is still a mystery
The article writes that for the pathogen of any epidemic, tracing the origin of the “primary culprit” It is vital to understand its evolution and prevent future epidemics. [ 96.2%), but the amino acid similarity of the receptor binding domain (RBD) of the spike protein that mediates the virus into human cells is only 89.2%. Immediately afterwards, multiple bat-derived coronavirus genome sequences that were highly similar to SARS-CoV-2 were also found in different countries.
The results of multiple studies have shown that SARS-CoV-2 is more likely to come from bats.However, the scientific community can determine that before bat-derived coronaviruses can acquire enough mutations to be able to infect humans, they need an intermediate host, just as the intermediate host of Middle East respiratory syndrome coronavirus is a dromedary.
Two previous studies have shown that SARS-CoV-2 related coronaviruses are found in Malay pangolins, and the RBD area is highly similar to SARS-CoV-2. However, compared with SARS-CoV-2, the overall genome similarity is low (<93%), indicating that pangolins are unlikely to be the intermediate host of SARS-CoV-2.
Gao Fu et al. took the study of the origin of HIV, HKU1 (HCOV-HKU1), SFTSV and MERS-CoV as examples, and proposed that tracing the origin of the virus requires long-term and extensive samples. It may take years or decades to accumulate. The geographic origin of the virus may have nothing to do with the initial patient. In some cases, “patient zero” may never be found.
Gao Fu pointed out that patients with similar symptoms before the pandemic should be reassessed, and samples stored in a wide range of areas should be retested. Blood banks and tissue banks are important resources for retrospective serological or genomics research , Especially in countries or regions where virus evidence has appeared in blood samples or environmental samples before the outbreak. In addition, it is necessary to clarify the natural host or intermediate host of the virus that requires a whole genome of animal samples that are susceptible to virus infection. Research.
The spillover effect of the virus
Gao Fu et al. wrote in the latest article that in addition to humans, several other mammals have been found Naturally infected with SARS-CoV-2 through contact with COVID-19 patients, such as zoosCats, dogs, lions and tigers, minks and ferrets in the Snow leopards, mountain lions and gorillas have also been found to be infected with SARS-CoV-2 in nature. 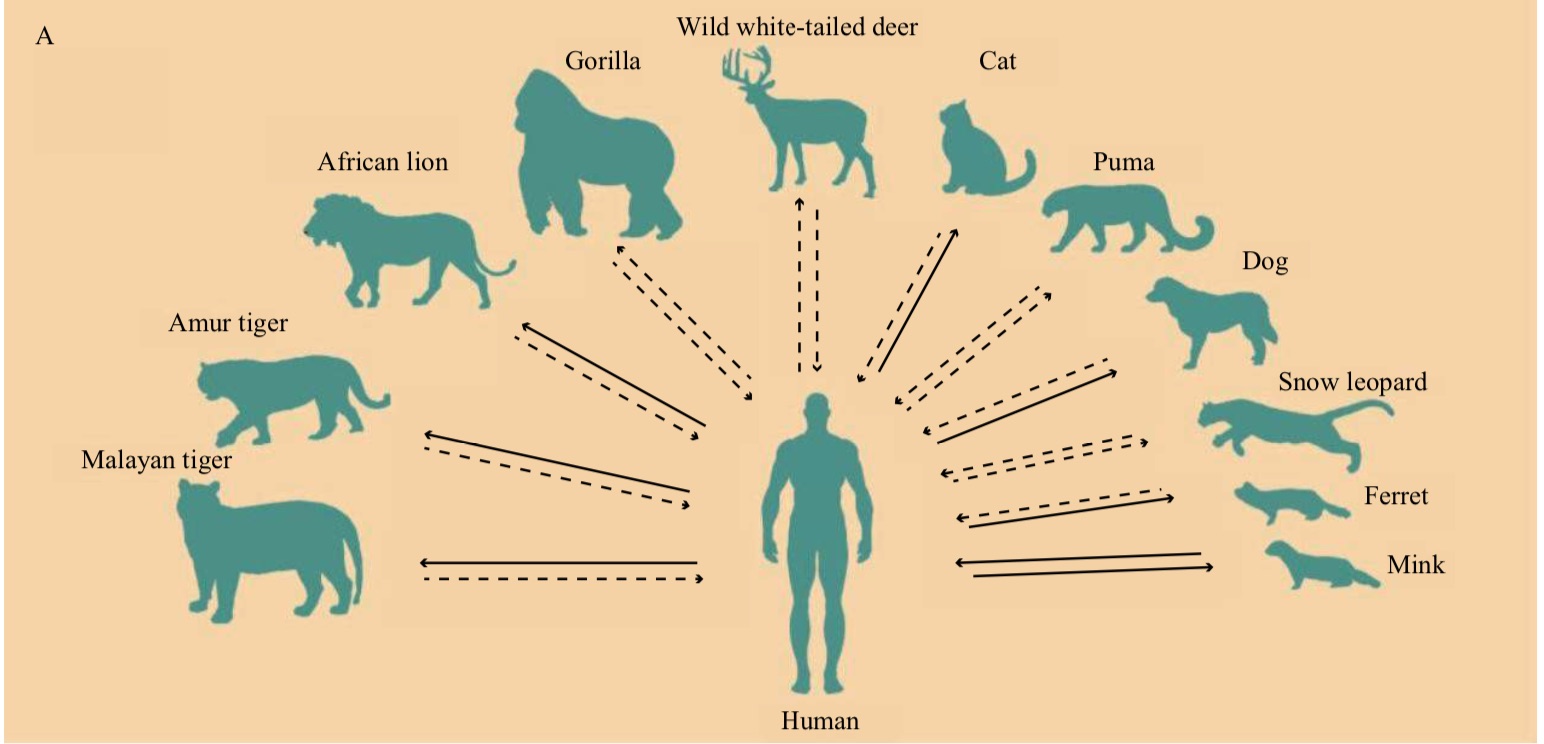 In these overflow events, the mink-related The SARS-CoV-2 variant has the greatest impact. Previous studies and related reports have made it clear that the mink has also transmitted the SARS-CoV-2 variant back to humans, leading to further community transmission. This also means that the spread of the virus between mink and humans has formed a “two-way transmission”.
In these overflow events, the mink-related The SARS-CoV-2 variant has the greatest impact. Previous studies and related reports have made it clear that the mink has also transmitted the SARS-CoV-2 variant back to humans, leading to further community transmission. This also means that the spread of the virus between mink and humans has formed a “two-way transmission”.
In addition to natural infection cases, infection experiments also show that a variety of animals may be susceptible hosts of SARS-CoV-2, such as rabbits, pigs, foxes, and fruits The raccoon waits.
Most of the above-mentioned natural infections occur in domestic animals, but far fewer cases have been found in wild animals. However, a recent serum monitoring in the United States showed that anti-SARS-CoV-2 antibodies were found in 40% of wild white-tailed deer (Odocoileus virginianus) samples in 2021. Antibodies were detected in 1 sample and 3 samples in 2019 and 2020, respectively.
Gao Fu et al. pointed out that although it is not clear whether the SARS-CoV-2 of wild white-tailed deer was introduced by humans, the sharp rise in the positive rate of antibodies indicates that SARS- CoV-2 has spread in wild white-tailed deer. Due to the wide geographical distribution of wild white-tailed deer in North America and the large number (about 30 million), the contact between humans and wild white-tailed deer can be achieved through wildlife restoration, field research, conservation work and some wildlife-related tourism, supplementary feeding, captive deer and Hunting and other activities to achieve. In this case, it increases the risk of SARS-CoV-2 from wild animals spreading to humans. 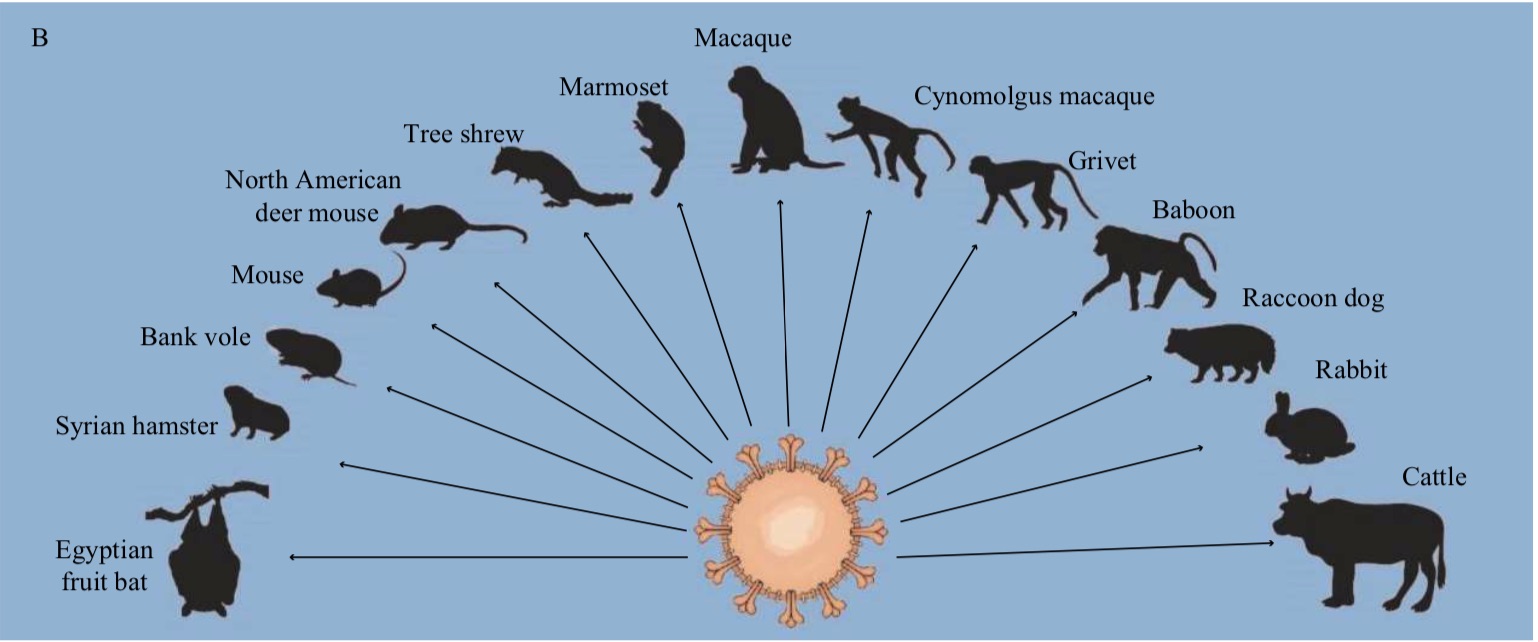 The article also analyzes the molecular mechanism. The mutations in several key sites of RBD and ACE2 of SARS-CoV-2 are closely related to the cross-species transmission of the virus. On SARS-CoV-2-RBD, sites 493, 498, and 501 were identified as key mutation hotspots that determine the host range.
The article also analyzes the molecular mechanism. The mutations in several key sites of RBD and ACE2 of SARS-CoV-2 are closely related to the cross-species transmission of the virus. On SARS-CoV-2-RBD, sites 493, 498, and 501 were identified as key mutation hotspots that determine the host range.
The 41 and 42 amino acids on ACE2 were identified as the key sites for SARS-CoV-2 RBD binding. There are 4 combinations (Y41-Q42, H41-Q42, Y41-E42 and H41-E42) at these two sites. Among them, the Y41-Q42 combination has the highest binding affinity for SARS-CoV-2 RBD. In addition, K31 and K353 have been reported to be mutation hotspots that can significantly change the free energy of binding. Mutations from K353 to A353 may disrupt the binding ability of ACE2 with SARS-CoV-2-RBD.
Host expansion is not over yet
At the end of the article, the host expansion of the coronavirus has been confirmed. A previous result showed that if some of the mutations that are adapting to mink are spread back to humans, and even trigger further community transmission, they will pose a huge threat to public health.
Because the mink is bred in the farm, large-scale slaughter of the mink can effectively prevent the spread of the mink source SARS-CoV-2 mutation and the accumulation of mutations in the mink population. However, similar measures cannot be taken for wild animals.
The article points out that when cross-species transmission occurs and spreads in new host populations, adaptive mutations are required, so more efforts are needed to investigate these in wild white-tailed deer. The genetic changes of the new variants and the corresponding spread and contagious effects on humans.
The article specifically pointed out that because SARS-CoV-2 is spreading to wild animals, many other wild animals will also be infected with SARS-CoV through direct or indirect contact with wild white-tailed deer. -2. Some experimental studies have shown that some animals may be susceptible to SARS-CoV-2, such as Egyptian fruit bats, common marmosets, macaques, bank voles and North American deer rats. However, these are only the tip of the iceberg, as most land wildlife has not been tested for susceptibility to SARS-CoV-2.
In addition, it is worth noting that the research on the susceptibility of marine wild animals (especially marine mammals) to SARS-CoV-2 is almost blank. Due to frequent human marine activities (such as marine aquaculture, marine fishing), the frequency of human contact with marine organisms is relatively high. If certain marine organisms are highly sensitive to SARS-CoV-2, there is a risk of human transmission to marine organisms.
More seriously, SARS-CoV-2 may spread in the marine ecosystem, resulting in some new SARS-CoV-2 mutations, posing an unknown threat to humans.
” Therefore, it is necessary to carry out large-scale SARS-CoV-2 screening for land and marine wildlife, especially susceptible wildlife, to monitor their infection According to Gao Fu and others, this also provides more clues for studying the origin and cross-species spread of SARS-CoV-2.