As the seventh coronavirus found to infect people, the new coronavirus (SARS-CoV-2) has been raging for more than two years. In the meantime, emerging variants continue to drive the global pandemic, and the need for more effective, broad-spectrum protective vaccines continues.  On March 31, local time, top academic journals A new study by a Chinese team titled “Circular RNA Vaccines against SARS-CoV-2 and Emerging Variants” was published online in Cell. ). This study is the first to report the circular RNA vaccine technology platform and the circular RNA vaccine developed against the new coronavirus and its series of variants.
On March 31, local time, top academic journals A new study by a Chinese team titled “Circular RNA Vaccines against SARS-CoV-2 and Emerging Variants” was published online in Cell. ). This study is the first to report the circular RNA vaccine technology platform and the circular RNA vaccine developed against the new coronavirus and its series of variants.
Qin Liang, postdoctoral fellow of Professor Wei Wensheng’s research group at Peking University School of Life Sciences, doctoral candidates Yi Zongyi and Shen Yong are the co-first authors of the paper, and the corresponding author of Wei Wensheng’s department. In addition, the research group of Professor Xie Xiaoliang/Researcher Cao Yunlong of Peking University, the research group of Professor Wang Jianwei of the Chinese Academy of Medical Sciences/Peking Union Medical College, the research group of Professor Peng Xiaozhong of the Institute of Medical Biology, Chinese Academy of Medical Sciences, the research group of Wang Youchun and Huang Weijin of the Chinese Academy of Food and Drug Control The research group has provided support.
In fact, the study was published ahead of time in the preprint bioRxiv in January this year, and compared to the bioRxiv paper the team published in March last year, they followed the mouse The circular RNA vaccine (circRNA^RBD) that they developed encoding the trimeric receptor binding domain (RBD) of the novel coronavirus spike protein was also verified in rhesus monkeys to elicit potent neutralizing antibodies and T cell responses.
The research team pointed out that the circular RNA vaccine (circRNA^RBD-Delta) against the delta variant of the new coronavirus prepared in this study is resistant to a variety of new coronavirus mutations The strain has broad-spectrum protection. They believe that the vaccine can be used as a new crown candidate vaccine with broad-spectrum protection, and also provides a reference for vaccine development and vaccination strategies against the current rapid spread of new crown variants. 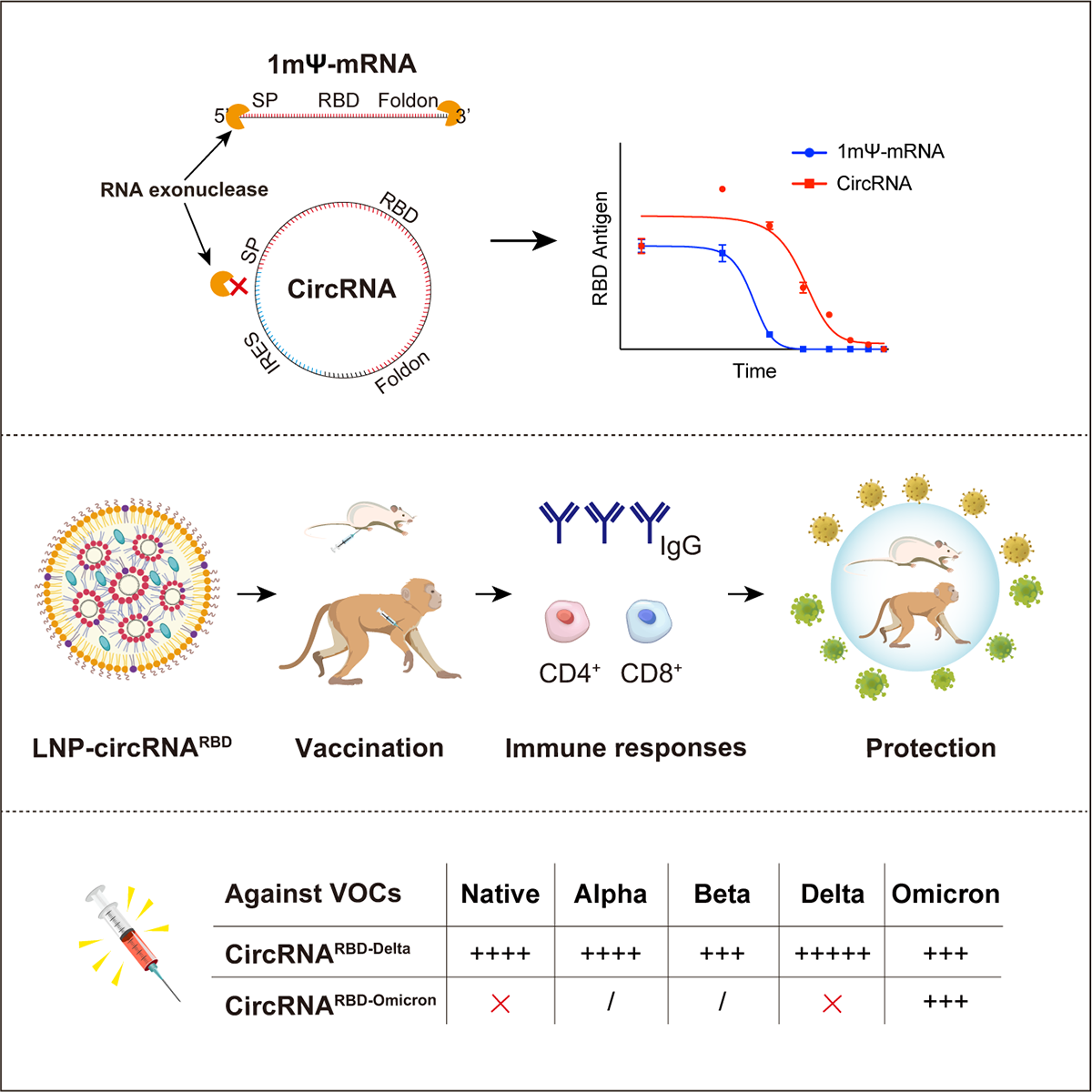
Schematic diagram of the development of circRNA vaccine for COVID-19.
Demand for new vaccines and constraints on mRNA vaccine technology
The paper wrote that with the development of the epidemic, mutations with immune escape ability appeared strains, the most severe of which is Omicron, which has now become the dominant strain in the global pandemic. It carries more than 30 mutations in the spike protein (S protein), 15 of which are located in the receptor binding domain (RBD), resulting in a significant decrease in the effectiveness of previously developed neutralizing antibodies.
The research team believes that although the neutralization ability of the original SARS-CoV-2 vaccine can be partially improved after booster vaccination, the Omicron pseudovirus has The neutralization capacity is still 4-13 times lower than wild type. This poses a serious challenge to the efficacy of existing vaccines, highlighting the urgent need to develop effective vaccines against this rapidly spreading variant.
Traditional vaccine platforms such as inactivated, virus-like particles have been used to develop COVID-19 vaccines with proven safety and efficacy. Of course, what has attracted the most attention in this epidemic is that mRNA vaccines against the new coronavirus have been developed at a breakneck speed and quickly approved for use. Before that, the vaccine development strategy was still in clinical trials and had never been used commercially.
As a breakthrough medical technology emerging in recent years, the basic principle of mRNA vaccine is to express antigen protein by introducing mRNA into the body through lipid nanoparticles (LNP) to stimulate The body produces a specific immune response. After the outbreak of COVID-19, Moderna’s mRNA-1273, Pfizer/BioNTech’s BNT162b2, such targeted mRNA vaccines stood out among a variety of vaccine types.
However, mRNA vaccines, which have the advantages of fast production speed, low cost, and rapid response to virus mutation, also have some shortcomings. For example, its storage and transportation conditions are harsh (minus 70°C) and have potential immunogenic side effects.
At the same time, Wei Wensheng also pointed out in a document provided to the reporter of the news (www.thepaper.cn) that the modification and delivery technology of mRNA vaccines were produced by foreign institutions , restricting the development and application of mRNA vaccines and their therapeutic technologies in my country, so there is an urgent need to develop newType and efficient vaccine technology.
Different from linear mRNAs, circular RNA molecules have a covalently closed circular structure without 5-Cap and 3′-polyA structures; and do not need to be modified bases, which are more stable than linear RNAs. Circular RNAs are a large class of non-coding RNAs, which are produced by a non-canonical splicing method called backsplicing. Discovered in 1976, it has become a new generation of star molecules in recent years.
In contrast, circular RNAs are highly stable due to their covalently closed loop structure, which protects them from exonuclease-mediated degradation. Circular RNAs were previously reported to be more stable than linear mRNAs, with a median half-life of at least 2.5-fold longer than linear mRNAs in mammalian cells.
However, to date, only a few endogenous circular RNAs have been shown to function as templates for protein translation. Natural circular RNAs generally cannot be translated because they do not have a cap structure at the 5′ end. However, it can achieve protein translation through an internal ribosome entry site (IRES) or by adding m6A modifications upstream of the open reading frame (ORF).
Therefore, the research team envisioned that circular RNAs could serve as a platform for generating immunogens. Wei Wensheng and others also pointed out that the circularization method and purification strategy of RNA are still immature, the impact of its potential immunogenicity on vaccine development is not clear, and many unknown factors also restrict the development and application of circular RNA.
Wei Wensheng told reporters that the research and development of his team’s circular RNA vaccine started after the outbreak.
The paper shows that the research team adopted the Group I ribozyme autocatalytic strategy to generate a circular RNA encoding the SARS-CoV-2 RBD antigen, called circRNA^RBD. To enhance the immunogenicity of RBD antigens, a signal peptide sequence (SP) was added to the N-terminus of RBD to achieve its secretory expression. In this structure, an internal ribosome entry site (IRES) element is placed before the RBD coding sequence to initiate its translation. To enhance the immunogenicity of RBD antigens, they also fused the signal peptide sequence of human tissue plasminogen activator (tPA) to the N-terminus of RBD to ensure antigen secretion.
In addition, given that the S protein trimer is better at binding hACE2The monomeric S protein, meanwhile, in order to improve the immunogenicity of the RBD antigen, the trimerization motif of T4 phage fibrin (Foldon) was fused to its C-terminus. The IRES-SP-RBD-Foldon sequence was then inserted into a circularized vector to construct an in vitro transcription (IVT) template for circRNA^RBD generation.
Preliminary display: the designed vaccine effectively neutralizes various mutant strains including the Omicron strain
Wei Wensheng et al mentioned that the experiment proved that , The circular RNA vaccine encoding the new coronavirus spike protein RBD can induce high levels of new coronavirus neutralizing antibodies and specific T cell immune responses in mice and rhesus monkeys, and can effectively reduce the incidence of new coronavirus infection in ganges. The viral load in monkey lungs significantly relieved the symptoms of pneumonia caused by new coronavirus infection. 
CircRNA vaccination provided significant protection in mice and rhesus monkeys.
A series of comparative evaluations showed that compared with mRNA vaccines, circRNA vaccines have the following characteristics or advantages: circRNAs have higher stability and can produce higher levels and more durable antigens in vivo; circRNA vaccines induce the body to induce The proportion of neutralizing antibodies produced is higher, which can be more effective against virus mutation and reduce the potential side effects of antibody-dependent enhancement (ADE) of the vaccine; the ratio of IgG2/IgG1 induced by circRNA vaccine is higher, indicating that it mainly induces Th1 type A protective T-cell immune response can effectively reduce potential vaccine-associated respiratory disease (VAERD) side effects.
Features and advantages of CircRNA vaccines (compared to mRNA vaccines).
In addition, it is worth mentioning that after the Omicron mutant was listed as a variant of concern (VOC) by the World Health Organization (WHO), the research team also urgently initiated a loop against the mutant. RNA vaccineR&D.
Within 30 days of obtaining the virus sequence information, the research team completed the entire process from vaccine production, mouse immunization to efficacy evaluation. The study found that the circular RNA vaccine (circRNA^RBD-Omicron) based on the Omicron variant has a narrow range of protection, and the antibodies induced by it can only neutralize the Omicron variant.
However, the circular RNA vaccine (circRNA^RBD-Delta) designed against the delta variant can induce broad-spectrum neutralizing antibodies in mice, and is effective Neutralize various new crown mutants, including the Omicron strain. They pointed out in the discussion that this circular RNA vaccine designed against the delta variant may be an effective booster option after existing vaccinations, and it is hoped that further trials will prove this. 
The circRNARBD-Delta vaccine designed for the new coronavirus delta variant is a candidate vaccine with broad-spectrum protection.
In addition, the research team also mentioned that although this research work did not specifically study the safety of vaccines or drugs, it is worth noting that circRNA vaccines were not used in non-human primates after vaccination in the study. Cause clinical symptoms or enhance pathology, which opens the way for the development of circRNA-based vaccines or drugs.
The research team concluded that the above results show that the circRNA^RBD-Delta vaccine designed for the delta variant of the new coronavirus is a candidate vaccine for new coronary pneumonia with broad-spectrum protection This study also provides a reference for vaccine development and vaccination strategies for the current rapid spread of new coronavirus variants.
At the same time, they believe that the establishment of this platform-based technology also has a wide range of applications in the prevention or treatment of infectious diseases, autoimmune diseases, rare diseases and cancer prospect.
Wei Wensheng said that the main follow-up work is to promote industrialization. It is worth noting that, according to previous reports on the official website of Peking University, Wei Wensheng’s team has successfully transformed circular RNA technology into productivity, and established Yuanyin (Beijing) Biotechnology Co., Ltd.Biotechnology Co., Ltd. focuses on the development of vaccines and novel therapeutics using circular RNA (circRNA) technology.
Thesis link: link: https://www.cell.com/cell/fulltext/S0092-8674(22)00394-4
