In the early morning of May 5th, Beijing time, the top medical journal “New England Journal of Medicine” (NEJM) published a study online by Gao Fu, director of the Chinese Center for Disease Control and Prevention and academician of the Chinese Academy of Sciences, which disclosed the internationalization of the protein subunit new crown vaccine ZF2001. Results of a multicenter randomized, double-blind, placebo-controlled phase 3 clinical trial.  ZF2001 vaccine contains SARS-CoV-2 receptor Binding domain (RBD) dimer and aluminum hydroxide adjuvant, the vaccine was jointly developed by Gao Fu’s team and Anhui Zhifeilong Kema Biopharmaceutical Co., Ltd. Previous Phase 1 and Phase 2 clinical trials have demonstrated the vaccine’s safety, acceptable side effects and immunogenicity in adults.
ZF2001 vaccine contains SARS-CoV-2 receptor Binding domain (RBD) dimer and aluminum hydroxide adjuvant, the vaccine was jointly developed by Gao Fu’s team and Anhui Zhifeilong Kema Biopharmaceutical Co., Ltd. Previous Phase 1 and Phase 2 clinical trials have demonstrated the vaccine’s safety, acceptable side effects and immunogenicity in adults.
This clinical trial ends in December 2021. The paper mentioned that the Phase 3 clinical trial was conducted under the circumstance that a new wave of SARS-CoV-2 variants dominates the global epidemic. Alpha hit in early 2021, but was quickly replaced by the delta variant in the following months.
In the Phase 3 clinical trial, the research team chose a three-dose vaccination regimen. The trial was conducted at 31 clinical centers in Uzbekistan, Indonesia, Pakistan, and Ecuador; in addition, only one clinical center in China was included in the safety analysis. Adult participants (age ≥18 years) were randomly assigned in a 1:1 ratio to receive 3 doses of 25 μg doses (30 days apart) of ZF2001 vaccine or placebo.
The primary endpoint was the onset of PCR-confirmed symptomatic Covid-19 at least 7 days after the third dose. A key secondary efficacy endpoint was severe to critically ill Covid-19 (including Covid-19-related death) at least 7 days after the third dose.
From December 12, 2020 to December 15, 2021, a total of 28,873 participants received at least 1 dose of ZF2001 or placebo and were included in safety Analysis; 25,193 participants who completed the three-dose regimen were included in the latest primary efficacy analysis at the second data cutoff date of December 15, 2021, with approximately 6 months of follow-up data.
Of all participants, 27,065 (93.6%) were 18-59, 60 and olderOf the 1839 (6.4%), 9383 (32.5%) were female. 78.5% of the participants were Asian and 17.7% were multiracial. More than 99.9% of the participants had not previously been infected with SARS-CoV-2 or vaccinated. At baseline, 13.2% of participants had other medical conditions.
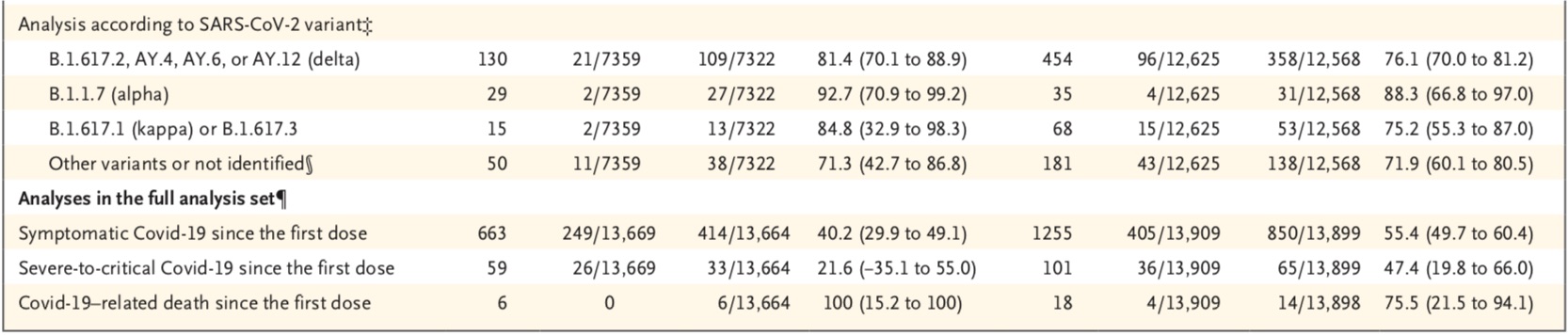
Short-term follow-up protection showed that at the first data cut-off date, 663 confirmed cases were in the first dose; 224 of these cases were at least Onset occurred 7 days later and was analyzed as the primary end point case. Of these 224 cases, a total of 36 cases occurred among the 7359 participants in the ZF2001 group, and a total of 188 cases occurred among the 7322 participants in the placebo group. The results of the analysis showed that the protective efficacy of the vaccine was 81.4% (95% confidence interval CI, 73.3-87.3).
A total of 14 cases met the criteria for severe to critical Covid-19 in the modified full efficacy analysis set. One case was in the ZF2001 group and 13 cases were in the placebo group. The vaccine was 92.9% effective (95% CI, 52.4-99.8). A total of five Covid-19-related deaths occurred, all in the placebo group. Among participants with disease with severe Covid-19 risk factors, vaccine efficacy was 84.4% (95% CI, 41.8-97.2). Vaccine efficacy was 81.2% (95% CI, 72.8-87.3) in participants aged 18-59, and 87.6% (95% CI, 2.5-99.7) in participants 60 and older .
Long-term follow-up protection showed that at the second data cutoff, 1255 Covid-19 cases were confirmed after the first dose of the vaccine. Of these confirmed cases, 738 had onset at least 7 days after the third dose and were assessed as the primary endpoint. Among them, 158 cases occurred in 12,625 participants in ZF2001 group, and 580 cases occurred in 12,568 participants in placebo group. The results of the analysis showed that the protective efficacy of the vaccine was 75.7% (95% CI, 71.0-79.8).
The vaccine was 87.6% (95% CI, 70.6-95.7) effective against severe to critical Covid-19, with 6 participants in the ZF2001 group developing Covid-19 -19 confirmed cases and 43 participants in the placebo group had confirmed cases of Covid-19. Vaccine efficacy against Covid-19-related deaths was 86.5% (95% CI, 38.9-98.5), ZF2001Two participants died in the group and 12 in the placebo group. Among participants with disease with severe Covid-19 risk factors, vaccine efficacy was 61.6% (95% CI, 29.5-79.9). Vaccine efficacy was 76.0% (95% CI, 71.2-80.1) in younger participants (18-59 years) and 67.6% (95%) in older participants (≥60 years). % confidence interval 21.9-87.8). 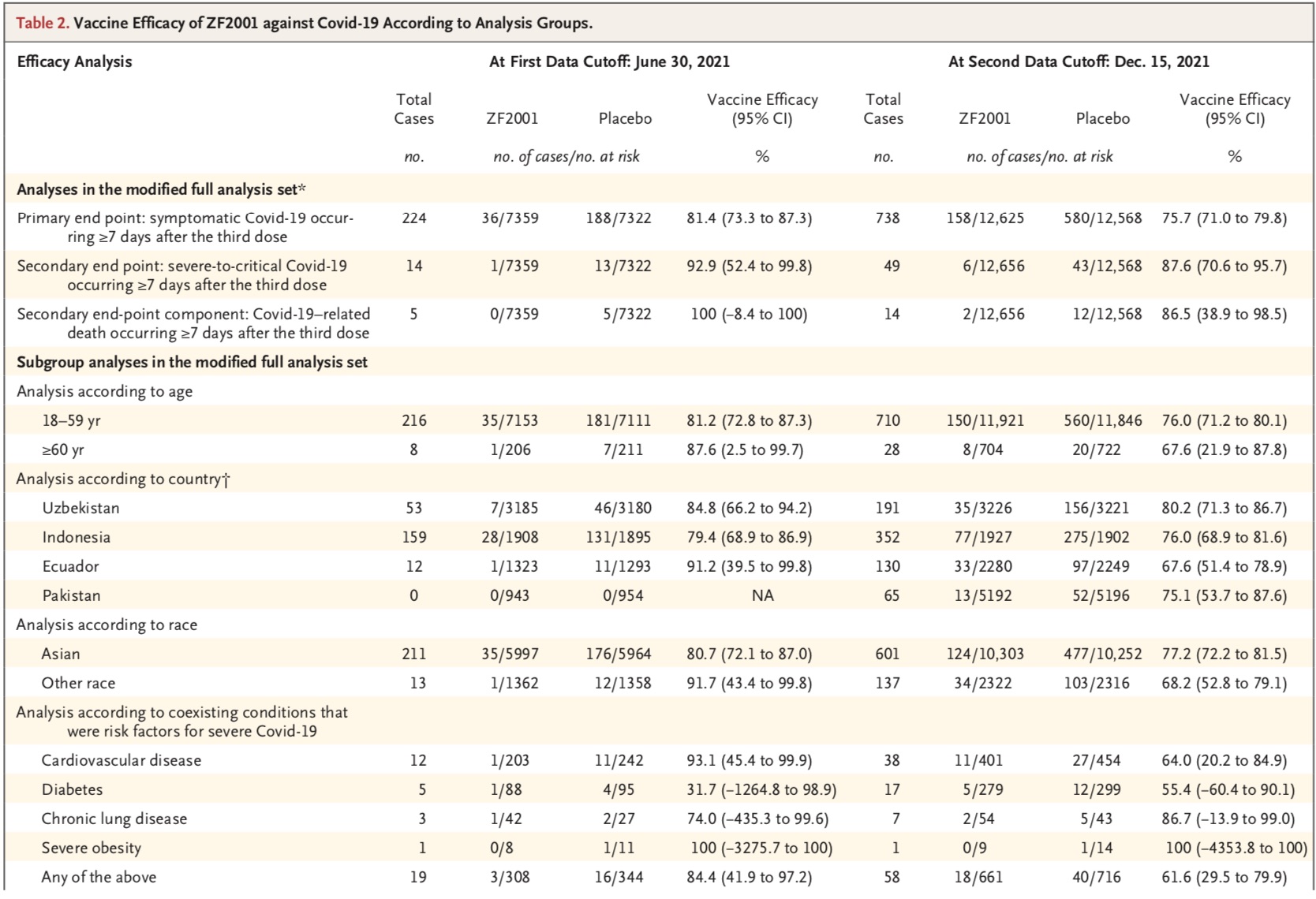
ZF2001 Analysis of vaccine protection against Covid-19.
In terms of safety, grade ≥3 adverse events in the ZF2001 group were relatively rare in both phase 2 and 3 trials (2.7% vs. 1.5%), and serious adverse events clearly related to ZF2001 were also rare (none occurred). vs. 2 onwards). Older participants (≥60 years) had lower rates of adverse events and reactogenic events than younger participants (18 to 59 years). The research team believes that these results show that the protein subunit vaccine using aluminum adjuvant has a good safety profile. 
Adverse events at the second data cutoff date (December 15, 2021).
It is worth noting that, given that the clinical trial was conducted under the leadership of the new coronavirus variant, the research team also analyzed the protective efficacy of the vaccine against different variant strains. Their results showed that ZF2001 was 92.7% effective against alpha variants in short-term follow-up and 88.3% in long-term follow-up; 81.4% effective against delta variants and 76.1% in long-term follow-up; targeting Kappa (B.1.617.1), the corresponding two data were 84.8% and 75.2%.
The research team concluded that in a large-scale adult cohort, ZF2001 vaccine is safe and can effectively prevent symptomatic and severe to critically ill Covid-19 for at least 6 months after full-course vaccination.
In fact, the vaccine has been approved for emergency use in China and Uzbekistan in March 2021, and it is the first recombinant protein vaccine approved for use in the world. It has also been approved for emergency use in Indonesia and Colombia. In addition, ZF2001 was approved for conditional marketing in China this year and as a sequential booster shot of the new crown inactivated vaccine.
The technical route of recombinant protein vaccine is one of the five new crown vaccine research and development technologies deployed in China. The research team believes that the recombinant subunit protein vaccine has the advantages of high yield, high safety, easy storage and transportation, and is one of the important options for the prevention of new coronary pneumonia.
The New England Journal of Medicine also published an editorial in the same issue, with Dr. Hanna Nohynek, chair of the Covid-19 vaccine working group of the World Health Organization Strategic Advisory Group of Experts, and coordinator In the article, Dr. Annelies Wilder-Smith pointed out that despite the global shortage of Covid-19 vaccines in 2021, by mid-2022, vaccine supply will no longer be a factor limiting equitable access to vaccines. As of April 19, 2022, approximately 11.5 billion doses of Covid-19 vaccine have been administered worldwide.  However, why do we need new Covid- 19 Vaccines? Nohynek et al. believe that each vaccine product has different characteristics and advantages and disadvantages, and that multiple factors must be considered when guiding policy decisions. Different countries and medical institutions, as well as different subpopulations and age groups, may benefit from different vaccine products developed on different platforms. When a country decides to procure and introduce a new Covid-19 vaccine, the outcome is not only the efficacy and safety evaluated in phase 3 trials. Other important considerations include the ease of implementation of the vaccination program, the effectiveness of the vaccine when used in routine programs, the need for and frequency of booster shots, cost, cold chain logistics considerations, scalability of production, population acceptance, and local or regional production range.
However, why do we need new Covid- 19 Vaccines? Nohynek et al. believe that each vaccine product has different characteristics and advantages and disadvantages, and that multiple factors must be considered when guiding policy decisions. Different countries and medical institutions, as well as different subpopulations and age groups, may benefit from different vaccine products developed on different platforms. When a country decides to procure and introduce a new Covid-19 vaccine, the outcome is not only the efficacy and safety evaluated in phase 3 trials. Other important considerations include the ease of implementation of the vaccination program, the effectiveness of the vaccine when used in routine programs, the need for and frequency of booster shots, cost, cold chain logistics considerations, scalability of production, population acceptance, and local or regional production range.
In their article, they believe that this ZF2001 by Gao Fu et al. and another plant-based coronavirus sample published at the same timeParticle vaccines, development of two new vaccines ‘to be commended’. They mention that both vaccines have the advantage of not requiring extreme cold chain storage conditions, making them easy to administer in primary care settings and in low- and middle-income countries, especially since phase 3 trials of both vaccines are in low- and middle-income countries. participate.
Nohynek and others also reminded that the first few Covid-19 vaccines put into use during the epidemic may not be the best long-term solution. Next-generation Covid-19 vaccines need to have broader epitope coverage, achieve longer duration of protection, and be easy to update in a timely manner to generate protection against new variants.
In addition, it is worth noting that for the new crown variant, the Gaofu team and the cooperative team are continuing to develop a new generation of recombinant protein vaccines. On April 26, the top international academic journal “Cell” published online the important progress of the research team in the field of new coronary pneumonia vaccine research. The research team developed a chimeric receptor binding domain ( RBD) dimer protein vaccine design method, which is formed by two heterologous RBDs in tandem. Compared with homologous RBD dimers, chimeric RBD dimers can stimulate the production of broader-spectrum antibodies in animals. react and provide better protection.  The prototype-Beta chimera designed by this strategy Mice and rhesus monkeys immunized with RBD dimer protein vaccine showed protection against multiple mutant strains in challenge experiments; the designed Delta-Omicron chimeric RBD dimer vaccine efficiently protected mice against Delta and Omicron infection and pneumonia.
The prototype-Beta chimera designed by this strategy Mice and rhesus monkeys immunized with RBD dimer protein vaccine showed protection against multiple mutant strains in challenge experiments; the designed Delta-Omicron chimeric RBD dimer vaccine efficiently protected mice against Delta and Omicron infection and pneumonia.