Original title: “We surveyed more than 30 medical AI imaging companies, and the industry’s development direction in 2020 is here.” span>
In 2015, medical artificial intelligence is on the rise. Engineers from all walks of life came to the medical field with their own algorithms, but found that the medical data was unexpectedly barren. They chose the field of lung nodules, which is relatively more operable, and began the initial development of medical artificial intelligence.
In the next three years, standardized medical data has gradually become full. Fundus, brain, heart, orthopedics, liver … more and more medical artificial intelligence maps are being built up little by little by researchers, gradually forming a web. However, with the newly-made nets, fishermen still have difficulty catching big fish, and the industry has entered into reflection.
There is indeed room for improvement in fishing nets. Equally important is the consideration of nurturing waters and the improvement of net casting methods.
What’s the problem? What to choose next? In this regard, Arterial Networks surveyed 31 imaging-related medical artificial intelligence companies, including Tencent’s search for images (Tencent) , and Ping An Smart City = “text-remarks” label = “Remarks”> (Ping An of China) , Xingmai Technology (Fosun High-Tech Holdings) Medical AI teams in large enterprises, all medical AI imaging companies after the B round and many non-head medical AI companies.
How does an artificial intelligence company make a web?
Before understanding the development path of artificial intelligence companies, let us first understand the current status of the development of artificial intelligence products at the end of 2019.
Extending from lung nodules, today’s artificial intelligence has now entered many departments such as cardiology, endocrinology, pathology, ultrasound, laboratory and other departments. They usually use the hospital where the company is located as the breakthrough point for product landing, and push forward when the product matures.
This advancement is manifested in the increasing coverage of artificial intelligence medical products, while moving across departments. The data in the statistical table, among the 31 companies participating in the survey, 21 companies have involved two or more departments, and their applications are increasingly diverse..
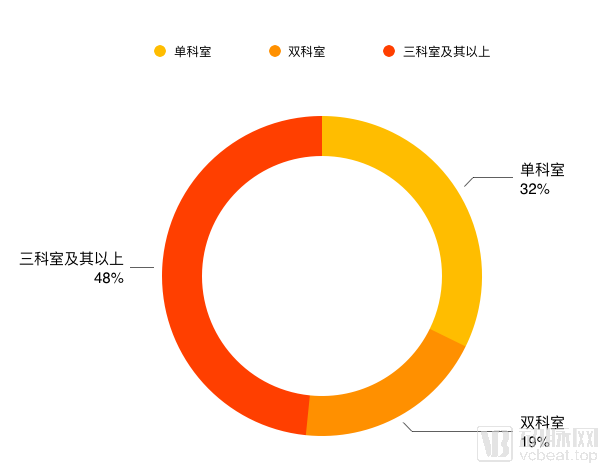
Department distribution
From the statistical data, the companies focusing on single departments are mainly image-assisted enterprises and radiotherapy-assisted therapeutic companies, such as Lianxin Medical and Datu Medical, which are particularly focused on this aspect. Enterprises are mostly in Pre-A round and A round.
The enterprises of the two departments are concentrated in the ophthalmology and pathology department. Most of the enterprises facing the two departments are not due to product expansion, but due to the needs of the disease. For example, sugar network diseases need to pay attention to both ophthalmology and endocrinology.
In this survey, there are many companies in the three divisions and above. The rounds B and above have matured and have the strength to overcome multiple divisions at the same time. The incubation team of listed companies focusing on Tencent’s video search and Ping An Smart Medical has a very strong strength in this aspect, and head start-ups such as Infertech, Itu Medical, and Shenrui Medical have also achieved multi-disciplinary offices. Cooperate.
The differences in the entry of AI companies into departments are reflected in the products. In general, the development of product lines in 2019 can be roughly classified into four roads.
First, the vertical layout of image companies, rapid development of modular products, and the formation of comprehensive solutions. Taking Shenrui Medical and Ande Medical Intelligence as examples, they have developed modules such as stroke, head and neck, and later merged the two modules to form a complete neural system AI solution.
Secondly, the image industry has gone deeper and deeper, and developed the past single disease AI to the whole disease. Taking SciTech and YiTu Medical as examples, both companies are trying to create a full range of lung cancer products that cover the needs of multiple clinical departments to create new demand.
Third, the whole process solution created by the radiotherapy company for a single scene. Taking Lianxin Medical as an example, its products cover target area mapping, automatic planning, radiotherapy quality control, and information management of radiotherapy departments. The entire product system is embedded in the workflow of doctors and physicists, providing them with comprehensiveDirectional help.
Fourth, the enterprise develops a scientific research platform for doctors to promote the cooperation of medical enterprises in scientific research. In this field, Shukun Technology, Yitu Medical, Inferential Technology, Shenrui Medical, and Huiyihuiying are all involved.
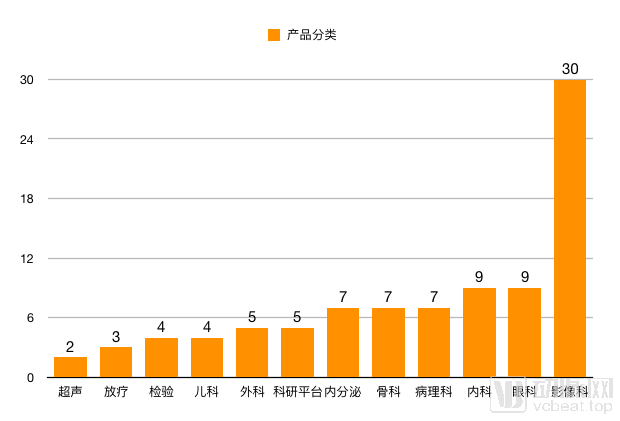
Research Product Categories
From the perspective of data, the way AI companies obtain data mainly comes from clinical data and scientific research data. Two years ago, medical data may only be called small and medium-sized data, but more than 10 companies in the table have processed millions of medical data, and the disease type is not limited to pulmonary nodules. This increase in information means that companies have more raw data and can conduct more in-depth research.
From the perspective of the landing hospitals, the top 500 hospitals in the top 500 are almost all covered by 31 artificial intelligence companies surveyed, and their acceptance of artificial intelligence has increased significantly. However, from the perspective of the winning information, the AI project in the strict sense has only won one bid. The target project is a hospital diagnostic image processing software procurement project with a purchase amount of millions of yuan. The rest of the winning information is mainly based on the cloud PACS system. It ranges from 6 million to 9 million. Cloud PACS sales account for most of the revenue of nearly 100 million-level artificial intelligence companies.
In addition, if we compare the increase in the number of companies entering hospitals between 2018 and 2019 and the increase in the number of companies entering hospitals between 2019 and 2020, we can find that the expansion of hospitals by leading companies throughout the year 2018 With more than 100, the growth rate of AI companies in the second echelon also exceeded 50. This rate has slowed down throughout 2019. The number of head companies has dropped to less than 100, and the increase of some non-head companies has been almost negligible.
The source of frustration or slowdown in commercialization. It is a well-known fact that it is difficult for medical AI to get money in 2019. In this case, blindly delivering artificial intelligence products to hospitals will actually cause the company’s operating costs to skyrocket; if it is only installed and not maintained, the hospital will lose trust in the company. So from this perspective, before commercialization has run through,The slowdown reflects the development status of the industry to a certain extent. If no products are approved in 2020, this data may show negative growth.
So where does the money go if there is n’t too much marketing? Scientific research is a good direction.
Assuming that medical artificial intelligence will open the road to commercialization in 2020, the core of the market grab between companies is still products. In this year, many companies have shifted their focus from sales coverage to research and development, and the results they have achieved are also amazing.
Although there are no exact figures, we can use several large data to see the contribution of companies in thesis: 2018 MICCAI corporate papers were included in more than 20 papers, 2019 more than 40 papers; 2018 RSNA There are 408 Chinese papers included, and 453 in 2019.
Many companies have received extraordinary results in their papers in specific academic conferences. MICCAI has included 8 papers in Tencent; 7 in Lianying Intelligence; 6 in science and technology; 5 in deep medical ; Zhiyuanhui Figure 4; Tuma Shenwei, Airdoc … RSNA included 17 papers of speculative technology … Many of them are clinical validation research papers.
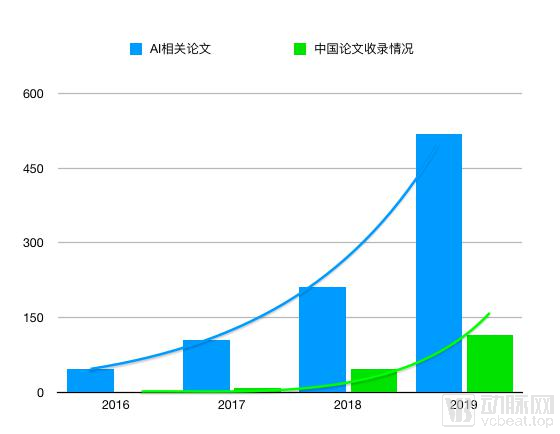
RSNA overall paper acceptance situation
What kind of fish breeds what kind of fish
To this day, the R & D logic of designing the product first and then looking for the demand scenario has failed. However, the attributes of the scene itself determine the development prospects of AI products. Therefore, the choice of scene determines the starting point of AI products.
Through the arterial network, the demand for artificial intelligence products can be broken down into the following figure.

Analyze the design logic of image AI products from the demand side
Look at the macro level first. Compared with the United States, we can find that the US imaging equipment market is becoming saturated, hospital management and supervision mechanisms are perfect, and doctors are facing an increasing number of patients and personalized diagnosis and treatment needs. Due to efficiency considerations, the hospital does not have much incentive to purchase new equipment, but is willing to upgrade the equipment by implanting software.
To a certain extent, this feature was demonstrated at the RSNA exhibition. As the host GE introduced new equipment, it also showed a large number of solutions to optimize the workflow of the imaging department. In view of the regional distribution of the United States population, Lianying exhibited PET / CT medical vehicles. These customized requirements may open new markets for manufacturers.
Domestic forms are very different. On the whole, there is a large gap in domestic medical resources, there is a lot of room for upgrading imaging equipment, and it can intuitively reflect the comprehensive strength of the hospital. The hospital has the power to purchase imaging equipment and use AI to supplement doctors’ manpower. In contrast, domestic artificial intelligence products are more concentrated in the field of auxiliary diagnosis.
Furthermore, the needs of different institutions in China are different. Large hospitals hope that AI can improve the overall operating efficiency of the imaging department, thereby shortening the length of hospitalization for patients; Basic hospitals hope to enhance the ability of doctors to diagnose and treat, leaving patients at the basic level; physical examination centers / third-party inspection centers Value artificial intelligence brings efficiency, accuracy, and project value added by AI; emerging eye centers, Medical Beauty Center hope to expand business scope through AI , add value to existing services; Doctors I hope that AI can improve work efficiency, and I also hope that companies will give their scientific research support.
So, in so many scenarios, which type of product can best meet the needs of doctors at this point in time? In the interview, Art.net learned that large hospitals, primary medical and physical examination centers / third-party inspection centers are most likely to land certain types of AI products in the shortest possible time.
For large hospitals, if an AI product can only improve the efficiency of reading doctors and make them get off work faster, thisObviously does not meet the interests of the hospital. Hospitals are hoping that doctors will report to patients more quickly, especially those who are already hospitalized, for clinicians to make faster decisions.
If the AI can reduce the two-day issuance time to half a day, the waiting time of hospitalized patients can be saved by 1 to 2 days, more patients can get treatment, and the total medical insurance expenditure of a single patient will be reduced. The hospital’s income will also benefit from this.
Such products are very demanding on the scene. Only scenarios where patients wait in line for treatment and large amounts of clinical data meet this requirement. From the perspective of the market, Coronary CTA has relatively mature AI products. Shukun Technology is the first to see this bright spot. Inferred technology and Shenrui Medical have also entered in 2019.
Primary care needs are different from hospitals. Under the grading diagnosis and treatment, primary care needs to better realize “minor illness does not leave the village”, it is necessary to improve the accuracy of diagnosis and treatment, and improve the ability of doctors to judge the patient’s condition. From the current form, many primary medical institutions need to improve “medical supply capacity”, not “improve efficiency.”
So, Many AI products that are deployed in basic-level hospitals need to be as operable and accurate as possible. But with the advancement of the medical consortium, the popularity of central reading formats, the possibility of terminal AI products entering the primary medical care becomes smaller, but AI CDSS that can provide accurate diagnostic paths may have greater applications space.
For the medical examination center / third-party imaging center, because the interests of the imaging department are more consistent with the interests of medical institutions, faster diagnosis and more accurate reports mean more income and better word-of-mouth. The value of intelligence will be more prominent. From 2018 to the present, the medical examination institutions such as Meinian and Aikang have all made enclosures on a platform, Ping An Health (Testing) , Hengdao Pathology Third-party inspection centers such as Panoramic Medicine have also developed AI products to improve efficiency.
Back to the imaging doctor’s perspective, their needs are less work time and more scientific support. Therefore, if the commercialization of AI is achieved through a good way with the imaging doctor, it is actually difficult to get through. However, imaging doctors can provide AI companies with the opportunity to enter hospitals, dig into existing product problems, correct the “Internet thinking” of AI developers, and provide scientific research data reasonably … Imaging doctors have become an integral part of the development of AI .
After understanding the situation of “fishing nets” and “waters”, the problems are also revealed. We can see that Most artificial intelligenceEnergy products are based on the needs of doctors and solve single-point problems faced by doctors. However, few artificial intelligence companies will tailor AI products for primary medical and physical examination centers. This choice can really satisfy doctors, but because the needs of payers have not been fully considered, the commercialization of AI has been hindered to a certain extent.
AI approval moves slowly
In addition to taking into account the needs of paying subject hospitals and using subject doctors; medical artificial intelligence also faces an old-fashioned fundamental problem-approval. For most of them, this problem left them in their throats-never fatal, but not immediately removed.
Over the course of 2019, there are only a handful of documents supporting AI in policy. From a national perspective, only on June 29th, the FDA issued relevant documents for review and approval of “Deep Learning Aided Decision-Making Medical Device Software Approval Essentials” to AI companies, and the “Industrial Structure” revised and issued by the National Development and Reform Commission on October 30 Adjustment guidance directory (2019 edition) “clearly mentions the promotion of medical artificial intelligence.
The establishment and improvement of medical databases has been gradual. It is reported that the medical image database led by the National Health and Medical Commission includes the ultrasound image library (40 diseases) , CT library, MRI library, etc. Hospitals or business units are of considerable size.
On July 15, 2019, only half a month after the release of “Key Points for Medical Device Software Approval for Deep Learning Assistance Decision”, the State Drug Administration Medical Device Technology Evaluation Center joined with the China Academy of Information and Communications Technology and Shanghai Shenkang Hospital to develop Center, Sichuan University, and many other institutions have established artificial intelligence medical device innovation cooperation platforms, and established the construction of at least 8 items including CT lung, CT liver, CT fracture, brain MRI, cardiac MRI, coronary CTA, ECG, ophthalmology Sample database.
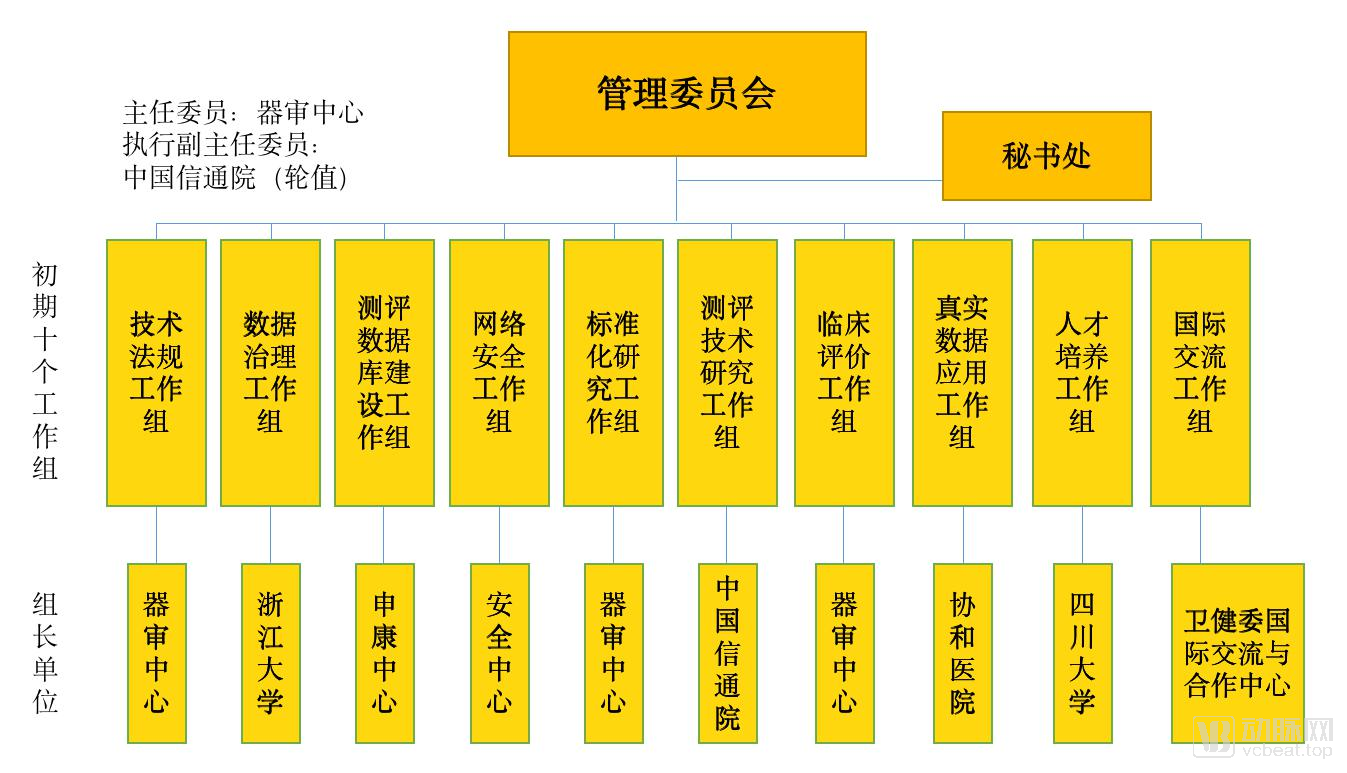
At the same time, the State Drug Administration, Shenkang Center and other institutions have also created specific evaluation platforms for artificial intelligence products, and their work operation methods are shown in the figure below.
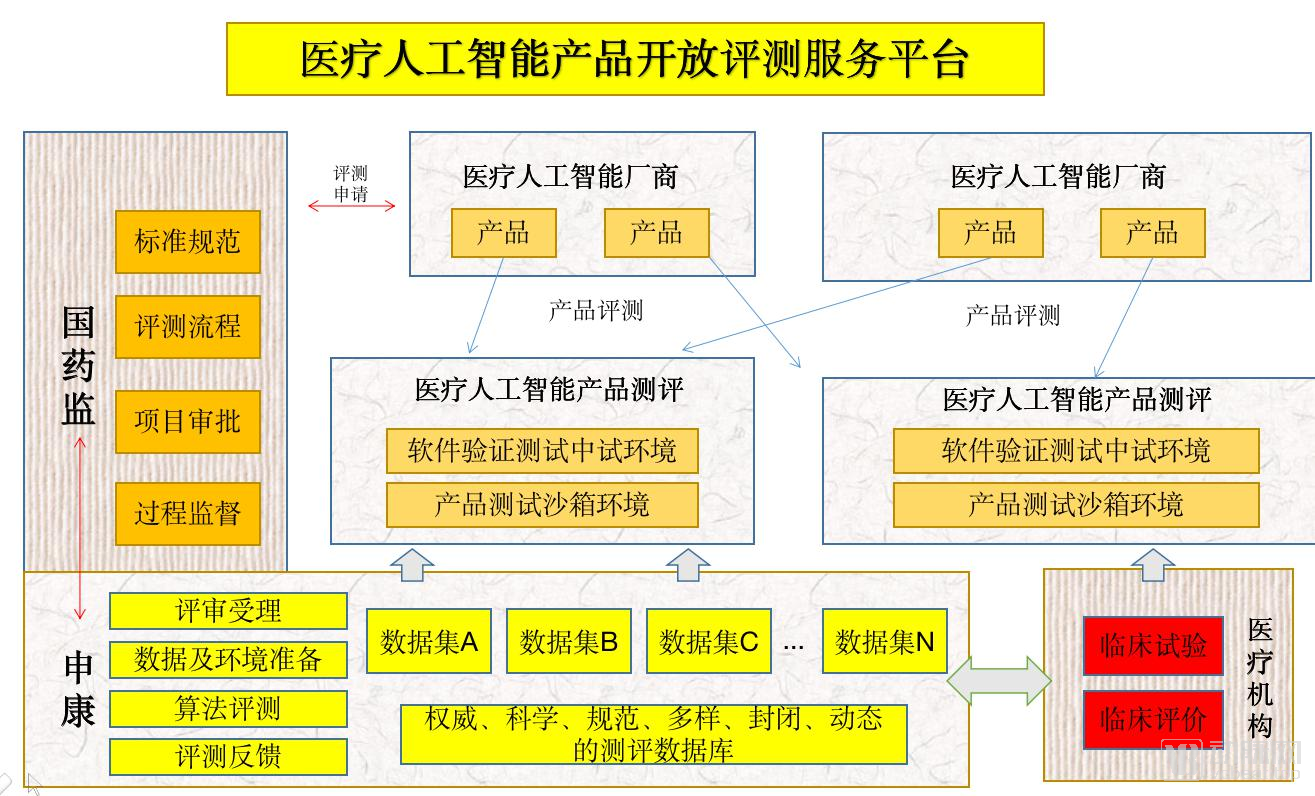
But even after confirming the approval process and division of labor, many companies are still stuck in clinical trials. As far as the radiology department is concerned, even mature products such as pulmonary nodules need to spend some time on clinical declaration and clinical trials to pass the approval, and other products still need to be polished.
As image reconstruction and image enhancement AI products do not involve auxiliary diagnosis, they can be sold with only type II certificates. Such products are one step ahead in the process of commercialization. According to statistics from January 2018 to September 2019, nearly 40 AI products have passed FDA approval, and half of them are non-assisted diagnostic products. For example, Shenshen Medical’s (Subtle Medical) SubtleMR, an AI platform for image reconstruction; GE’s mobile intelligent X-ray equipment for ICU has serious problems The “Critical Care Suite Optima XR240amx” has received FDA Class II approval through 510 (k) .
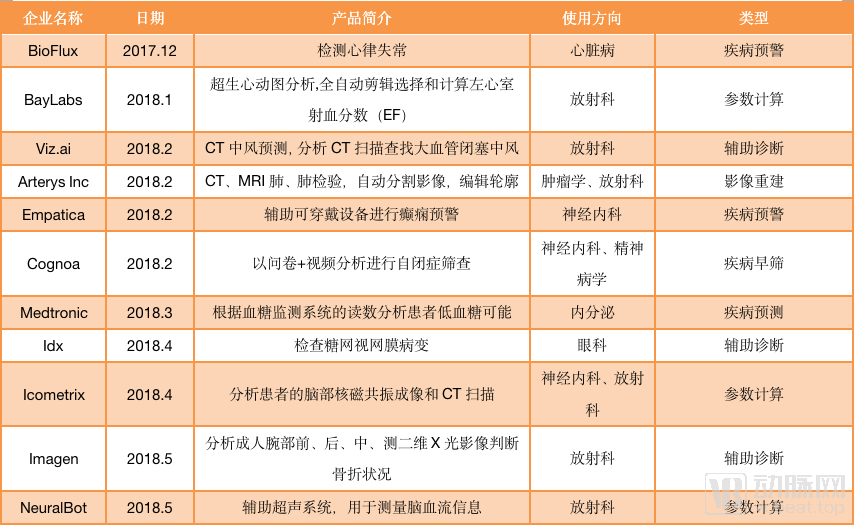
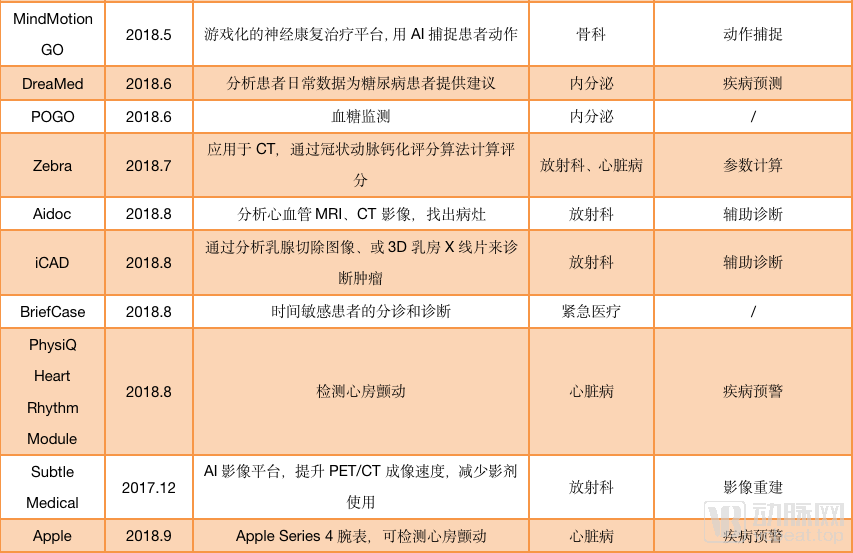
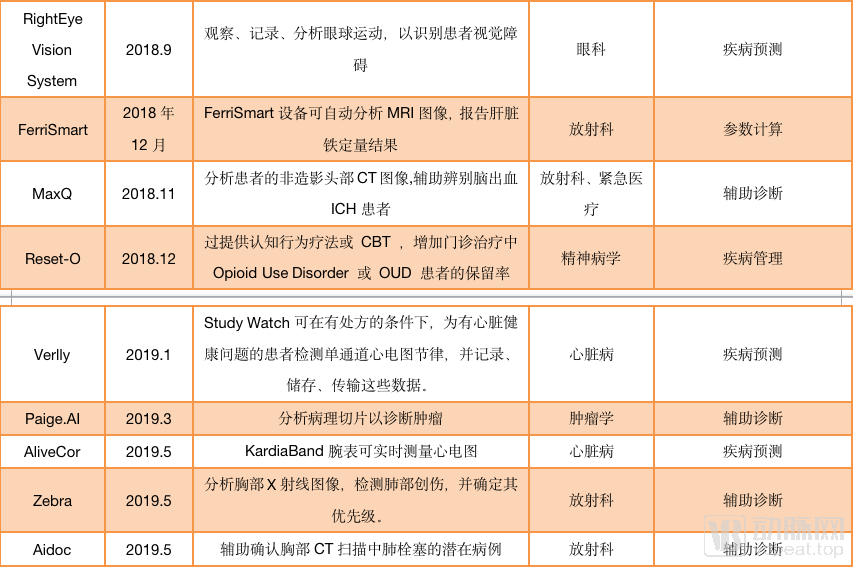

AI products approved by the FDA (statistics are from December 2017 to September 2019)
And among the FDA-assisted diagnostic products, many products under the auxiliary diagnostic label, such as Viz.ai, Imagen, more emphasis on the “warning” function of AI than the “diagnostic” function. For AI self-diagnosed products, only IDx-DR under IDx has passed FDA certification.
Businesses that focus on radiotherapy are different. Baiyang Technology, Lianxin Medical, Datu Medical and other smart products used for assisted radiotherapy have obtained Class III device certificates issued by NMPA, but these products do not have a clear definition of “smart”. If you need to rely on deep learning to assist in surgical planning and automatically generate corresponding reports in the future, you still need to pass the Class III device approval for “AI function”.
Hidden opportunities in collaboration
Overall, 2019 is not friendly to artificial intelligence, with less policy promotion, capital injection difficulties, and calm social cognition. Fortunately, innovations in algorithms such as federal learning, deep learning automation, and universal representation learning From the aspect of technology, it promotes the further application of AI.
What are the future prospects of imaging AI? In addition to continuing to enter the hospital according to the existing model, the arterial network has found two possible forms.
Trend 1: Cooperative imaging equipment manufacturers
Compared to the stagnation of the number of AI track companies, platform products have increased this year. From this year’s RSNA exhibition, we can find that in addition to GPS, Terrarecon, a large image data processing manufacturer, exhibited the envoyAI platform. Nuance, a clinical voice manufacturer, and former film giant Fujifilm also released their own AI platforms. >
These platforms often integrate the artificial intelligence developed by imaging equipment and equipment. Arterial Network has statistics on the current AI development of various companies. The content is as follows.
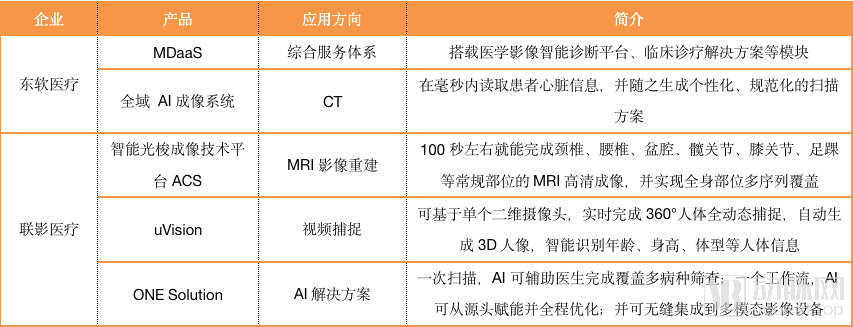
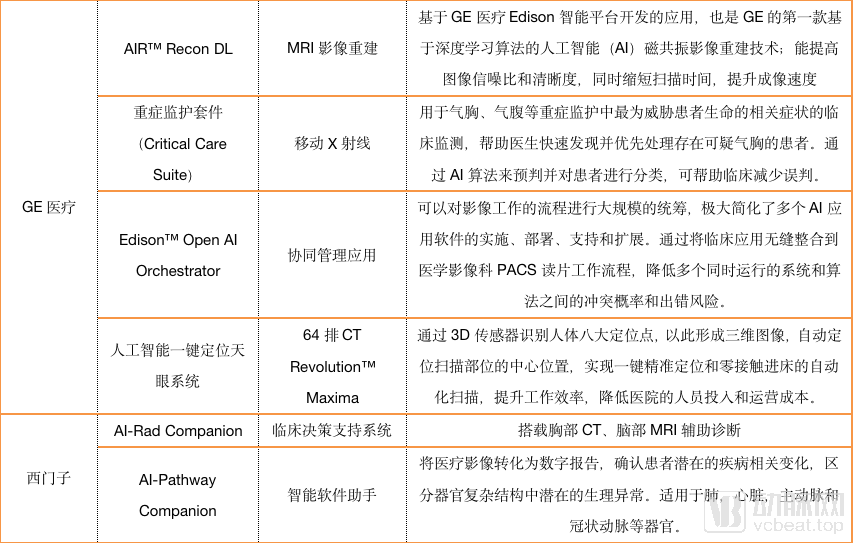
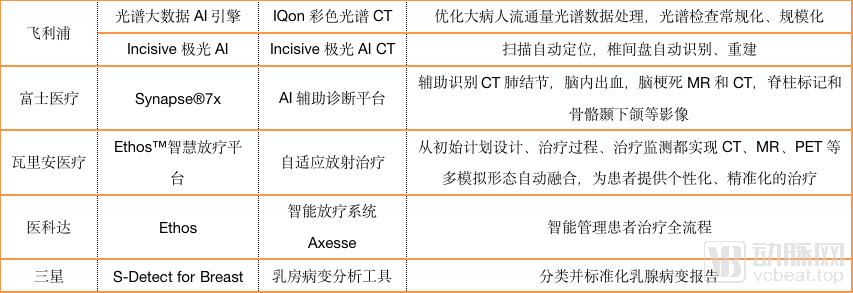
As can be seen from the chart, most imaging equipment companies have not focused on developing artificial intelligence for specific diseases, and GPS has established its own artificial intelligence ecosystem in China.
Lianying Medical and Varian Medical are the exceptions. Lianying Intelligent, a subsidiary of Lianying Medical, has undertaken the task of developing full-stack and full-process artificial intelligence applications; Varian has devoted itself to creating a full-process, multi-modality and personality. And precise adaptive radiation therapy.
If “AI-assisted diagnosis and treatment” is not a false proposition, then device manufacturers have left room for AI startups to develop. If AI startupsIf the highest quality artificial intelligence products can be opened at the lowest possible cost, then equipment manufacturers and hospitals may become their payers.
Trend 2: Cooperation with pharmaceutical companies through disease management
At the Expo, Novartis and Tencent released the nation’s first artificial intelligence disease management platform for heart failure diseases. Tencent’s artificial intelligence technology thus bridged the gap between pharmaceutical companies.
For Novartis, the data generated by Tencent’s consultation platform has great value. Through the integration of these data, Novartis can accurately understand the prevalence of diseases in Chinese residents.
AI companies can also build similar platforms. Many imaging companies are providing cloud PACS services to hospitals, and using this to build platforms and even cut into follow-up sessions to help patients with disease management. Through this process, they can also obtain trending data.
Take diabetic patients as an example. Patients are checked at least once a year, which means that patients will remain active on the chronic disease management platform for a long time. Such a platform is very attractive for pharmaceutical companies.
Of course, in addition to these two trends, artificial intelligence still has other directions, such as cooperative medical examination centers, providing services directly to the C-side … As long as effective requirements can be discovered, these directions have value.
However, if a group of artificial intelligence companies can pass the approval of three types of devices in 2020, the pattern may be very different. This can be regarded as the common demand of the entire industry. I hope no one will become a “walker”.