This new type of battery can be cut, bent, immersed, fired and even ignited with fire, and it can still discharge. New technologies, new perspectives, and new trends.
Editor’s note: Lithium-ion batteries are widely used in various portable mobile electronic devices with their unique performance advantages. Large-capacity lithium-ion batteries have also been used in the field of electric vehicles, and will even be used in satellites, aerospace and energy storage. However, in the past few years, news about the explosion of Samsung Note7 mobile phones has been frequently reported. The main reason behind it is also the flammability of lithium-ion batteries. A few days ago, “WIRED” published an exciting news, the original title of the article was “Scientists Made a Nearly Invincible Lithium-Ion Battery”, and the author DANIEL OBERHAUS introduced an article made by American scientists New impregnable lithium-ion battery.
Image source: GETTY IMAGES @CASEY CHIN
Li-ion batteries have almost reshaped the entire modern world. Whether it is a mobile phone, a laptop, an electronic cigarette, or an electric car, it is a core element of its rechargeable electronic components.
Although lithium-ion batteries have better energy storage and high energy density, this does not mean that they are the perfect choice. Because lithium-ion batteries must be manufactured with toxic and flammable materials, even the smallest defects can cause the relevant equipment to explode.
A group of researchers composed of physicists at the Johns Hopkins Applied Physics Laboratory believes that the existing lithium-ion battery safety issues are fully capable of further improvement.
For the past five years, they have also been working on a new lithium-ion battery that appears to have no safety issues. In 2017, they, together with researchers from the University of Maryland, introduced a new lithium-ion battery that can cut, shoot, bend, and even soak, while maintaining its hold.Continue discharge.
At the end of 2019, the Johns Hopkins University team also made further improvements and upgrades to make this battery fireproof and raise its voltage to a level comparable to that of equivalent commercial products. Of course, this may also make Samsung jealous.
Konstantinos Gerasopoulos, a senior scientist responsible for this research at the Applied Physics Laboratory, said that to make this indestructible battery, the core lies in the electrolyte, which is the positive A chemical mixture that acts as a conducting ion between the negative electrodes.
When you are using a lithium-ion battery, the charged lithium particles will pass through the obstruction of the electrolyte, from the anode (negative electrode) at one end of the battery to the cathode (positive electrode) at the other end, and produce a chemical reaction and energy in it.
Most of the lithium-ion electrolytes are a mixture of flammable lithium salts and toxic liquids, said Jeff Maranchi, materials science project manager at the Applied Physics Laboratory, which means “the lithium ion today There is a potential disaster risk in the chemical industry. ”
If the permeable barrier that separates the anode and cathode of the battery is damaged, it will cause a short circuit and generate a lot of heat. When all this heat comes into contact with the extremely flammable materials (such as lithium ion electrolyte) next to the oxygen-rich cathode in the battery, you will have a burning electronic device in your hand.
Aqueous batteries can avoid all of these problems. After all, the electrolyte is inherently aqueous, so it is neither flammable nor toxic. Although water-based batteries have been around for about 25 years, their efficacy is too weak to be effective.
What the Applied Physics Laboratory has discovered is that if they increase the lithium salt concentration and combine the electrolyte with a polymer (a material that looks like an extremely soft plastic), they can move the potential from 1.2 volts Increase to 4 volts, reaching the same level as commercial lithium-ion batteries.
When Grassopoulos and his colleagues connected the anode and cathode of a commercial battery with this plastic-like electrolyte, they successfully created a lithium ion that has never been seen on the market. battery.
This battery is transparent, has the flexibility of contact lenses, and is non-toxic and non-flammable. Whether it is made or used, it can be carried out in the open without a casing. In addition, it can withstand almost any kind of “ill-treatment”.
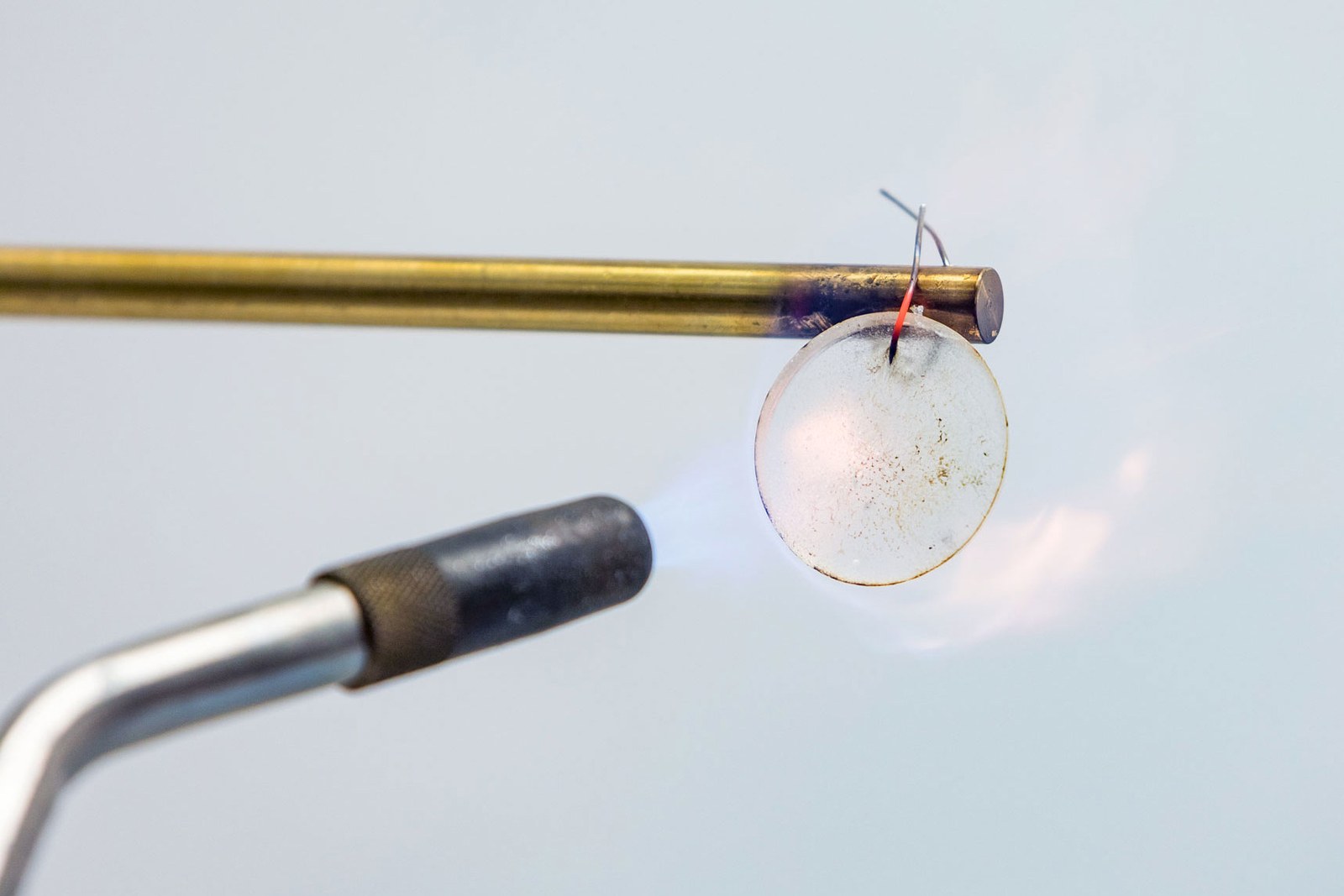
The electrolyte of the new battery core is a mixture of lithium salt and a soft plastic material that is not easy to burn or explode. Image source: JOHNS HOPKINS APL
During the test for this battery, the Applied Physics Lab team tried immersing the sample in salt water, cutting it with scissors, using an air cannon to simulate ballistic impact, and finally igniting it with fire. During each test round, the battery kept discharging normally. After one of the tests was ignited by fire, the experimenter removed the charred part, and the remaining part was still used normally for about 100 hours.
Malanchi also said that this new type of water-based battery does not only exist and is in the laboratory. His team is already in contact with multiple unidentified manufacturers. These manufacturers say they can easily incorporate this new chemistry and form factor into existing lithium-ion battery generation lines. According to Marantz, it can be put into the commercial market in two years, and can even be used in areas that have not been involved before.
Because of its flexibility, it can also be integrated into wearable electronic devices, and even finally be directly implanted into clothing fibers.
Its strength and durability advantages also suggest new uses in many military and technological applications, such as unmanned underwater vehicles, drones, and satellites.
Of course, there are some technical obstacles to this battery, such as increasing the charging cycle of water-based batteries. Generally speaking, the battery of a smart phone can achieve more than 1,000 charge and discharge times, but this new type of battery will lose potential energy after the charge and discharge times reach 100 times.
Glassopoulos says fine-tuning the electrolyte’s chemistry may provide new solutions.
By then, potentially explosive electronic devices will cease to exist.
Translator: Junyi