Shuanghuanglian experienced a night of magical robberies, and then rose to a daily limit on A shares.
文 梦 家 菜菜
Edit Hong Hong
On February 3, the A-share market opened. Of the total 3,792 companies in Shanghai and Shenzhen, 3,185 stocks had their daily limit. In a cloud of misery, a group of “Shuanghuanglian concept stocks” ushered in a head start against the market.
Take Harbin Pharmaceutical Group, the parent company of Shuanghuanglian Oral Liquid Leading Company, Harbin Pharmaceutical Co., Ltd., whose performance and stock price have been slumped, as an example. According to its 2019 performance forecast, net profit for this year will be The “78% -90%” decline, but with the “double Huanglian concept”, Harbin Pharmaceutical Co., Ltd., along with a group of Shuanghuanglian manufacturers such as Fusen Pharmaceutical, Tailong Pharmaceutical, Lukang Pharmaceutical, and Daan Gene, welcomed the entire line Daily limit.
The sentiment for Shuanghuanglian was passed to the capital, which is almost a predictable ending. The time should be set back 4 days ago, January 31st- This is the 12th day after Zhong Nanshan first confessed to the public that “(new coronavirus pneumonia) must be transmitted by people” in the CCTV interview. Late at night, Shuanghuanglian suddenly ushered in its “Magic Night”.
Originally, social media hotspots on this day included: In the early hours of the day, the WHO announced the global outbreak of the new crown virus epidemic as an “public health emergency of international concern” (PHEIC) because of inefficiency and the use of charity The mysterious and controversial Hubei Red Cross, as well as Han Hong’s charitable concept of “a pack of instant noodles can be publicized” in sharp contrast to it.
For most Chinese netizens, the focus of discussion on the epidemic bombing and continuous migration in the past few days is undoubtedly exhausting, but at 22:46 in the evening, one was issued by Xinhua Viewpoint and the official Weibo of People’s Daily. The news constituted the last sensational news in this constant day.
It is reported that “A preliminary study of the Shanghai Institute of Materia Medica and the Chinese Academy of Sciences of Wuhan Academy of Sciences found that the Shuanghuanglian oral liquid can inhibit the new coronavirus”. Affiliated Tongji Hospital conducts clinical research. ” As soon as the news came out, Changlong was rushing to buy Shuanghuanglian oral liquid in front of many drug stores. People temporarily put down the strategy of “home at home” and wore masks to line up in the middle of the night. On the e-commerce platforms such as Tmall and JD.com, within one hour of the news, almost all brands and stores of Shuanghuanglian oral solution showed that they were sold out. Even the “Veterinary” version of Shuanghuanglian Oral Liquid was also swept away, and the Shuanghuanglianrong moon cakes were shot inexplicably and magically driven by sales due to their similar pronunciation.
But at the same time, it was an online wave of doubts about the effect of “Shen Yao” Shuanghuanglian. The preparation and quality control of proprietary Chinese medicines are always controversial because they are not as strict as western medicines in the process. In 2017, the country has issuedSimplifying the Regulations for the Administration of Registration Examination and Approval (Draft for Soliciting Opinions) “proposed that the traditional Chinese medicine compound preparations derived from ancient classics should be exempted from clinical verification when applying for a drug approval number, which would further aggravate the dispute.
Some people also pointed out that Shuanghuanglian is also “top-ranked” in the adverse reactions of proprietary Chinese medicines. In the national annual report on adverse drug reaction monitoring from 2013 to 2014, Shuanghuanglian (oral liquid, granules, capsules, tablets) were all adverse. The vanguard of reactions: ranked second in adverse reactions in 2013 and first in 2014. Its adverse reactions mainly include gastrointestinal damage, skin and accessory damage, and even central and peripheral nervous system damage. However, after review, from 2015 to 2018, according to the official website of the State Drug Administration, the adverse reactions of Shuanghuanglian mixture were not outstanding, and the Shuanghuanglian mixture was not included in the annual report.
The Shanghai Institute of Pharmacy, the Chinese Academy of Sciences, which has always been professional, has also been turned out of “black history”: During the SARS period in 2003, the Shanghai Institute of Pharmacy stated that “the highest concentration of Jieyin lotion has certain resistance. The effect of SARS virus activity “was recorded in an article entitled” Anti-SARS Magical Use of Jieeryin “in the 13th issue of Capital Medicine. At the end of 2019, the Shanghai Institute of Materia Medica also announced the development of a new drug for the treatment of Alzheimer’s disease, claiming that “the Phase III clinical trial has been completed”, and a report letter signed by Rao Yi pointed out that academic fraud—strangely, biological scientists Dr. Rao Yi admitted afterwards that the letter was written by him, but he just stored the letter in the draft mailbox and did not send it.
However, after a night of plundering and questioning, in the early morning of February 1, the People’s Daily official issued a new statement, emphasizing that “the discovery of (Shuanghuanglian can inhibit the new crown virus) is still a preliminary study” and reminded the public “Do not snap up Shuanghuanglian Oral Liquid.” According to the WHO, “so far, there are no drugs for the prevention and treatment of the new crown virus”.
In the evening, Mr. Jiang Hualiang, Director of Shanghai Institute of Pharmaceutical Research also made a clarification , Said “only virus tests have been done, further clinical trials are needed.” In fact, in the process of drug development, no clinical trials have been conducted like this, and no clinical results have been seen. It is impossible to talk about the effectiveness of the drug on the new coronavirus in the human body.
This is a magic night of Shuanghuanglian. But if we look at each outbreak of an epidemic or epidemic, we will find that almost all Chinese patent medicines will be pushed to the front desk, treated as “magic drugs” for a short time, and sold out :
In 2003, the dean of China College of Traditional Chinese Medicine claimed thatLangen was effective in the treatment of SARS. After that, Banlangen was defeated in the first battle. By 2009, pandemic influenza and bird flu in 2013, Banlangen had been regarded as the “magic medicine” for treating all diseases. There are even rumors that it is possible to treat Ebola virus with extremely high lethality;
Unfortunately, during the period of pandemic, Lianhua Qingwen Capsule also used the concept of Chinese medicine to suppress the virus, and sales increased;
During the SARS period, Jieeryin was “confirmed” by the Shanghai Pharmaceutical Research Institute to be antiviral;
In the bird flu and influenza A period, honeysuckle, forsythia, windbreak, firewood, etc. are recommended for the prevention of influenza …
Although more rigorous controlled trials are yet to be carried out, after each epidemic, it is difficult to find any information to prove that these drugs are effective, but it will not affect the appearance of a proprietary Chinese medicine in the next epidemic. Random, selected again became popular.
The so-called “Shuanghuanglian inhibits new crown virus” is still in its initial discovery stage, and its effectiveness cannot be discussed yet. However, in our opinion, when there is an emergency, without proper scientific evidence, Chinese medicine—and any other medicine—should not be easily pushed to the altar. Chinese medicine needs time to test its clinical effect, and Chinese medicine cannot become a vanity market.
How far is the distance from “virus test” to “practical application”?
Let’s tease out every aspect of drug development. For a new drug, the industry often says “a billion dollars in ten years” to describe the time-consuming and costly.
The development of new drugs is divided into four parts: First, the discovery of new drugs is mainly to select diseases and targets, find and optimize lead compounds, and the time is uncertain. The second step is pre-clinical research. At this stage, animal experiments and pharmacological studies are usually performed, which takes 3-6 years. The third step is clinical research. What this link is doing is the effect of experimental drugs on the human body, including pharmacokinetics, pharmacology, toxicology, effectiveness, etc. Stages I, II and III usually take 6-7 years. The fourth step is registration approval and post-market inspection, which usually takes six months to two years.
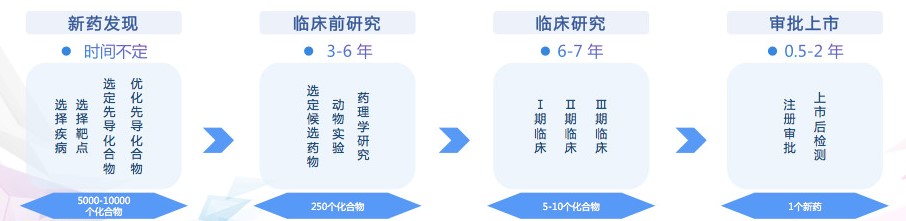
Shuanghuanglian’s “old medicine new use” is actually an additional indication. In this case, the first step of new drug discovery is generally omitted. Academician Jiang Hualiang said that “only virus experiments were performed, and further clinical trials are needed.”The toxic activity test shows that it is only in the second step of the picture, that is, the preclinical research stage.
Let’s take another look at the official Weibo of People’s Daily. “The drug is being clinically researched in Shanghai Public Health Clinical Center and Tongji Hospital affiliated to Huazhong University of Science and Technology.” . Because Shuanghuanglian Oral Liquid has been on the market for many years, we speculate that there is a probability that pharmacokinetic, pharmacological, and toxicological experiments will be omitted and the effectiveness will be directly tested. In this way, the clinical time can be greatly shortened.
Suffice it to say, Shuanghuanglian’s “old medicine new use” and clinical research and registration approval are not completed. What is the final success rate? Horizontal reference: From January 2006 to December 2015, there were 9,985 clinical trials (mainly western medicines) carried out worldwide. Such improved new drugs, from clinical stage I to final approval, had a success rate of only 22.6% (although this data Has a high success rate in various drugs).
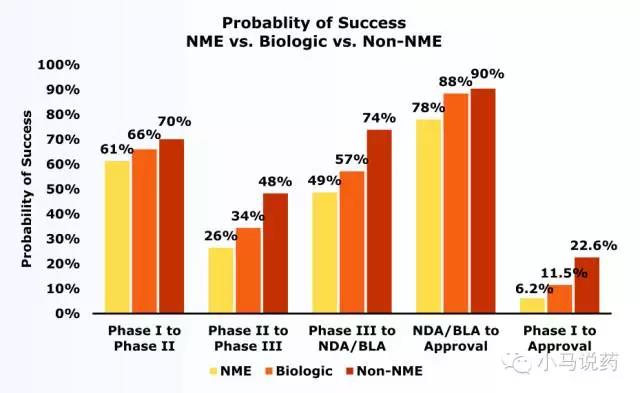
R & D success rate (pictures from the public account Xiao Ma said medicine)
NME: new molecular entity, 505 (b) (1) pathway; Non-NME: Non-new molecular entity, 505 (b) (2) pathway
It can be seen that the short word “preliminary discovery” represents a long distance, which means that there are still many meticulous clinical experiments that have not yet been implemented, and the application is really far behind.
Of course, in an emergency epidemic situation, related policies may be adjusted to help shorten research and development time. For example, it is possible to give green channels accelerated approval, or to give priority to re-examination and then make experiments.
On the evening of February 2nd, the Drug Evaluation Center of the State Food and Drug Administration just released an announcement saying, “During the epidemic, if there is a declaration of special products to prevent and control the new coronavirus infection pneumonia, the applicant can contact the Center Contact “, the latest news to help expedite filing.
Huge market affected by “Double Huanglian Night”
The plunder of Shuanghuanglian Oral Liquid has directly led to the denitration of major e-commerce platforms, and physical drug stores have also sold out.
There are 12 companies that are qualified to produce and sell Shuanghuanglian oral solution, involving 4 listed companies-Tailong Pharmaceutical, Harbin Pharmaceutical, Fusen Pharmaceutical, and Treasure Island.
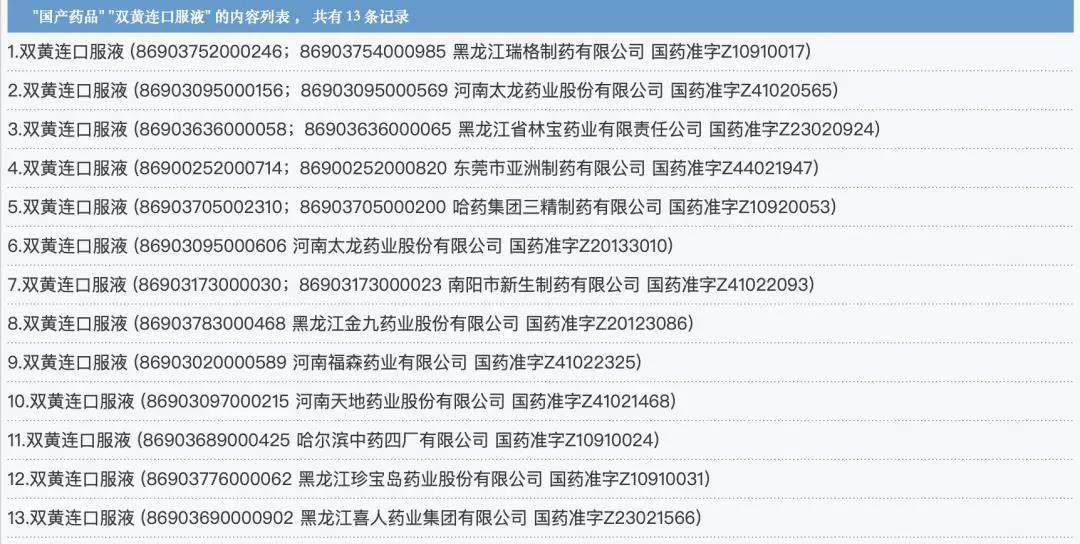
Information comes from the official website of the State Drug Administration, organized by Southern Metropolis Daily
On February 3, A shares opened in various anxieties about the epidemic. Tailong Pharmaceutical, Harbin Pharmaceutical, and Treasure Island have all reached daily limit. Hong Kong stock Fusen Pharmaceutical once rose by over 200%.
Interestingly, although the media report endorsed by Shuanghuanglian was released on the evening of January 31, as early as the 23rd, Shuanghuanglian oral liquid was officially included in the new coronavirus prevention program by the Beijing Administration of Traditional Chinese Medicine. On the 29th, it was included in the second edition of the New Coronary Pneumonia Prevention Program in Beijing.
As a result, some companies have begun to work overtime to stock Shuanghuanglian oral solution. On the 23rd, Tailong Pharmaceutical’s WeChat account stated that on the eve of the Spring Festival, they worked overtime to organize production and coordinate reserve transfers. On the 26th, Harbin Pharmaceutical Group started construction. On that day, Sanjing Pharmaceutical produced 1.3 million Shuanghuanglian oral liquids. Fusen Pharmaceutical also urgently deployed employees during the Spring Festival holiday, and all production lines are working at full capacity.
In the development of new drugs for the new crown
In fact, in addition to Shuanghuanglian oral liquid, there are many drugs in the “primary discovery” stage, but Shuanghuanglian oral liquid took the lead in the controversy. For example, a week ago, the joint research team of the Shanghai Institute of Materia Medica and the Shanghai University of Science and Technology of the Chinese Academy of Sciences announced the discovery of 30 Drugs that may have a therapeutic effect on new pneumonia .
Compared to the “hyped” Shuanghuanglian, it may be worth paying attention to the drug Remdesivir being developed by American Gilead. Also on January 31, Shuanghuanglian was snapped up, the New England Journal of Medicine reported the first 2019-nCoV patient in the United States, Remdesivir was started on the 7th day of hospitalization, and the fever and symptoms were reduced the next day. Science magazine also suggested that the combination of remdesivir and monoclonal antibodies is likely to be the ideal therapy for 2019-nCoV. Remdesivir is also mentioned in the 30 possible compounds mentioned above for treating new coronary pneumonia.
On February 3, Academician Zhong Nanshan stated thatThere are at least 7 small-molecule drugs targeting viral RNA polymerase or protease, including CR3022 antibody drugs, which are in different clinical research stages; research and development of related vaccines are also underway.
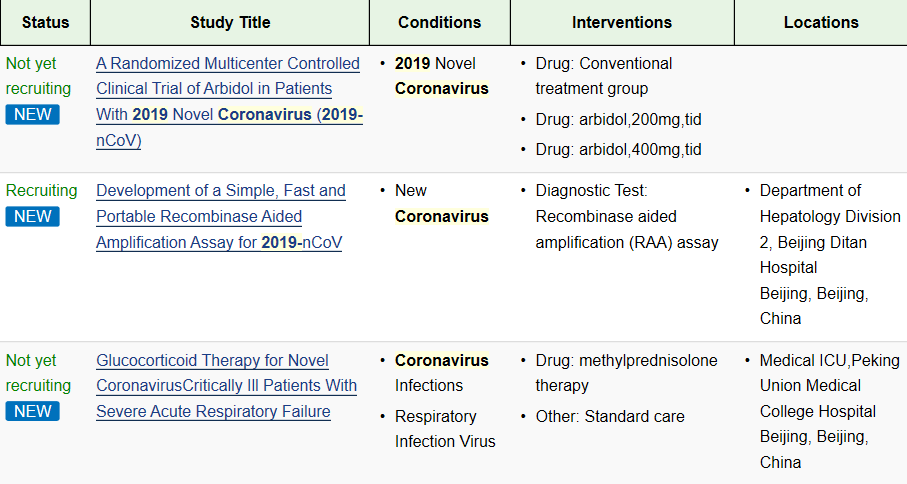
New crown virus research currently being performed globally
For the epidemic, human beings do not have the so-called idea to defeat the virus. They always developed scientific therapies after the stubborn battle of human beings, and they were eventually let go of the epidemic.
As the WHO said, there is currently no specific medicine to deal with the new crown, but we must believe that after a rational and scientific fight, we will see the dawn of hope.