The wide applicability of the therapy to cancer types also means that more patients can benefit from it.
According to foreign media reports , biotechnology company Xilio Therapeutics (formerly known as Akrevia Therapeutics) has completed over $ 100 million in Series B financing, led by Takeda Ventures, and is jointly participated by other new and old investors. This round of financing will further advance its candidate drugs, XTX201 and XTX101, into the clinical development stage, while expanding their selective tumor cytokine development pipeline. Previously, this company completed a $ 30 million Series A financing in 2018.
Xilio Therapeutics was founded in 2016 and is headquartered in Weihao, Massachusetts, USA. According to the official website, “Xilio” (pronounced “Ex -il-ee-oh”) is derived from the Latin word Ex Nihilo, meaning “creation” or “big bang”, and it embodies the company’s vision-to release tumors by releasing The full power of selective, highly effective immunotherapy to create transformative therapies for cancer patients.
Xilio Therapeutics’ president of research and development, Dr. Tim Clackson, said that Xilio’s IO therapy can block the binding activity of molecules as they flow through the body. Once they encounter proteases in the tumor, the blocking module is removed, releasing the therapeutic moleculeBiological activity. The broad applicability of this treatment to cancer types also means that all patients are expected to benefit from these potential treatments.
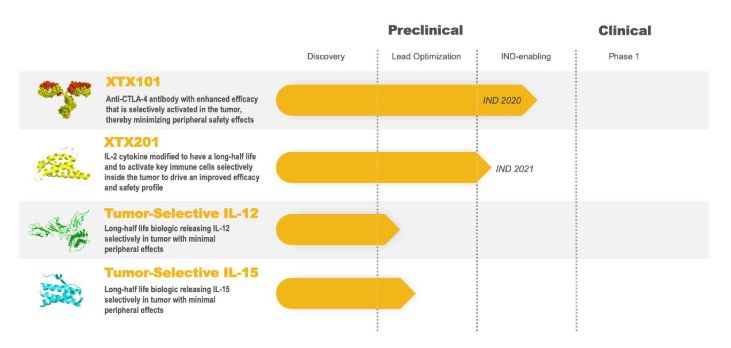
Xilio’s R & D pipeline (Image source: Xilio Therapeutics official website)
Specifically, IO therapy can be selectively activated in tumors to overcome the significant toxicity associated with effective IO treatments, such as IL-2 and aCTLA4, which limit the number of patients that can be treated and prevent The patient completes the full course of treatment.
At present, its lead drugs have demonstrated their effective selective antitumor activity in preclinical models. Among them, XTX201 (targeting IL-2) can induce tumor immune activity, but does not have the cytotoxicity of non-selective IL-2 treatment. Compared with ipilimumab, XTX101 (targeting aCTLA4) also significantly expanded the potential therapeutic indicators of these therapies and showed better antitumor activity.
This also indicates that selectivity to tumors significantly broadens the potential therapeutic index of these therapies. It is reported that Xilio Therapeutics plans to submit IND applications for these two drugs this year and next.