There are multiple drug candidates.
It is learned that Pliant Therapeutics , a fibrotic disease drug research and development enterprise, has announced that it has completed a US $ 100 million Series C financing. Novartis, led by Redmile Group, Farallon Capital Management, Cormorant Asset Management, Surveyor Capital and Logos Capital.
Dr. Bernard Coulie, President and CEO of Pliant Therapeutics, said: “This financing will support the clinical trials of our main candidate products in the treatment of IPF (idiopathic pulmonary fibrosis) and PSC (primary sclerosing cholangitis). Development, and ongoing strategies for a proprietary candidate pipeline for widespread fibrotic disease. “
The data shows that fibrosis is a pathological feature of many diseases, and it is due to the human body’s repair tissue Damage caused by abnormal natural abilities. If left untreated, fibrosis can cause scarring of vital organs, resulting in irreparable functional damage and even organ failure. Organs that may be fibrotic include lungs, liver, kidneys, heart, digestive tract, and more.
In response, compounds that are being produced by Pliant Therapeutics’ prolific drug discovery engine may address fibrosis in a variety of organs and diseases, including muscle (Du’s and other muscular dystrophies), liver (PSC, NASH, and Cirrhosis), kidneys (renal fibrosis), skin (scleroderma), and gastrointestinal tract (stenosis Crohn’s disease).
Public information shows that Pliant Therapeutics’ drug development strategy is to target two key mechanisms leading to fibrosis : TGF-β activation and epithelial-intercellular Epithelial-to-mesenchymal transition (EMT). Among them, TGF-β is a key factor that regulates pathological fibrosis, and EMT is the main reason that leads to the transformation of normal epithelial cells into activated fibroblasts. Pliant targets the TGF-β signaling pathway by inhibiting integrin to specifically antagonize the TGF-β signaling pathway in fibrotic tissues.
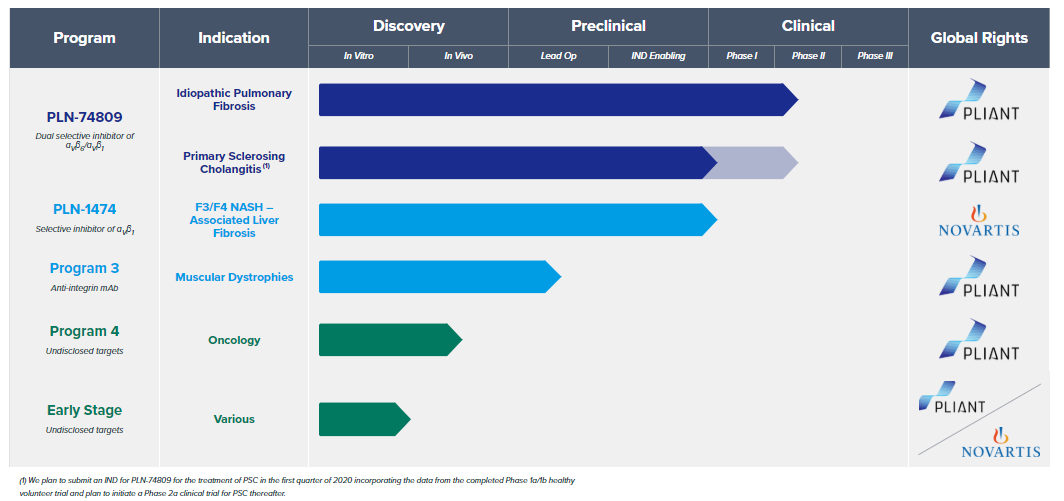
Pliant’s R & D pipeline (Image source: Pliant’s official website)
At present, the main drug candidate of Pliant is PLN-74809, which is a dual small molecule selective inhibitor capable of selectively inhibiting αvβ1 and αvβ6 integrins at the same time. Plays a key role in the transformation approach.
According to the research and development plan, this therapy will first be used to treat patients with IPF (idiopathic pulmonary fibrosis) and PSC (primary sclerosing cholangitis).
Learned , IPF It is a chronic, progressive fibrotic disease that thickens, stiffens, and forms scar tissue in the lung tissue, causing patients with progressive dyspnea and reduced quality of life. With the progress of fibrosis, it will become more and more difficult for the lungs to transport oxygen to the bloodstream, eventually leading to the failure of vital organs to obtain the oxygen necessary for normal physiological activities. 60% -80% of patients die within five years after diagnosis. Currently, The disease affects approximately 140,000 Americans. PSC is a chronic hepatobiliary disorder. It is characterized by diffuse inflammation and fibrosis of the biliary system leading to deformation of the bile ducts, and often has multiple stenoses. The disease progresses progressively, eventually leading to bile duct obstruction, biliary cirrhosis and liver failure.
The results of preclinical studies show that PLN-74809 can change the function of specific integrins expressed only in fibrotic tissues, thereby blocking TGF-β activation in a targeted manner to prevent or reverse lung and Growth of fibrotic tissue in the liver.
PLN-74809 is currently in Phase 2a clinical research. It has been awarded the Orphan Drug Designation by the US FDA in 2018 . (FDA orphan drug eligibility program aims to motivate and promote rare diseases-United StatesDiseases with fewer than 200,000 patients-development of drugs. This qualification will provide Pliant with a variety of business and incentives, including market exclusivity and tax deductions for clinical research fees, to address unmet needs for IPF patients. )
At the same time, Pliant also has a second drug candidate, PLN-1474, which is an oral selective αvβ1 small molecule inhibitor, mainly for nonalcoholic steatohepatitis (NASH). Related liver fibrosis.
References: PLIANT THERAPEUTICS RAISES $ 100 MILLION IN SERIES C FINANCING TO ADVANCE THERAPIES FOR FIBROTIC DISEASES