Since the outbreak of New Coronary Pneumonia COVID-19, more and more experimental teams around the world have joined the source tracing team to try to understand how the new coronavirus that caused the outbreak was transmitted from animal hosts to humans. As of now, the epidemic has caused more than 1.9 million diagnosed patients worldwide and more than 120,000 deaths.
It is generally accepted that the new coronavirus may be derived from bats, and its similarity to the RaTG13 sample collected by a team of researchers from Shi Zhengli, Wuhan Institute of Virology, Chinese Academy of Sciences in 2013 in Yunnan, China 96.2%. However, scientists are still looking for an intermediate host between bats and the first introduction of the new coronavirus into humans. Snakes and pangolins have all been included on the “suspicious list.”
The latest study adds another member to the list-dogs. On April 14, local time, the international authoritative journal “Molecular Biology and Evolution” (Molecular Biology and Evolution (its 2018/2019 impact factor is 14.797)) published a study online, giving a new speculation: dogs may It is an intermediate host that transmits the new crown to humans. The virus may evolve in the intestines of dogs, thereby gaining the ability to infect humans.
 There is only one author of the study and the corresponding author of the paper. It is Xuhua Xia, a professor of biology at the University of Ottawa, Canada. The official website shows that the research of Xia Xuhua’s laboratory focuses on vertebrate mitochondria as a simplified biological system with three basic biological processes; host-parasite interactions at the molecular level, especially the control of new ones through genome recombination and horizontal gene transfer Mechanisms of origin of viral and bacterial pathogens; evolution of microbial genomes; origin and evolution of alternative splicing; development of powerful computing tools.
There is only one author of the study and the corresponding author of the paper. It is Xuhua Xia, a professor of biology at the University of Ottawa, Canada. The official website shows that the research of Xia Xuhua’s laboratory focuses on vertebrate mitochondria as a simplified biological system with three basic biological processes; host-parasite interactions at the molecular level, especially the control of new ones through genome recombination and horizontal gene transfer Mechanisms of origin of viral and bacterial pathogens; evolution of microbial genomes; origin and evolution of alternative splicing; development of powerful computing tools.
 There is only one author of the study and the corresponding author of the paper. It is Xuhua Xia, a professor of biology at the University of Ottawa, Canada. The official website shows that the research of Xia Xuhua’s laboratory focuses on vertebrate mitochondria as a simplified biological system with three basic biological processes; host-parasite interactions at the molecular level, especially the control of new ones through genome recombination and horizontal gene transfer Mechanisms of origin of viral and bacterial pathogens; evolution of microbial genomes; origin and evolution of alternative splicing; development of powerful computing tools.
There is only one author of the study and the corresponding author of the paper. It is Xuhua Xia, a professor of biology at the University of Ottawa, Canada. The official website shows that the research of Xia Xuhua’s laboratory focuses on vertebrate mitochondria as a simplified biological system with three basic biological processes; host-parasite interactions at the molecular level, especially the control of new ones through genome recombination and horizontal gene transfer Mechanisms of origin of viral and bacterial pathogens; evolution of microbial genomes; origin and evolution of alternative splicing; development of powerful computing tools.
Xia Xuhua concluded that the results of his research provided a hypothesis for the origin and initial spread of the new coronavirus. First, the ancestors of the new coronavirus and bat-derived coronavirus RaTG13 infected the intestinal tract of a mammal (such as bat meat eaten by canines or humans). Second, the strong selectivity of canine enterovirus RNA genome for CpG may lead to the rapid evolution of the virus. A large number of mutations from CpG to UpGDue to the reduction of ICpG and GC% in the group. Third, dogs lick the anal area during mating and other conditions, helping the virus spread from the digestive system to the respiratory system. Finally, the reduced viral genome ICpG allows the virus to evade the human ZAP-mediated immune response and become a serious human pathogen.
He emphasized that this shows the importance of monitoring SARS-like coronavirus in wild dogs in the fight against the new coronavirus.
However, Xia Xuhua ’s inference has now caused many scholars to openly question it.
Antiviral sentinel: ZAP
Antiviral sentinel: ZAP
Wild mammal species including bats constitute coronaviruses (including SARS, MERS and the deadly new crown Virus). The paper mentions that different hosts or host tissues provide different cellular environments, especially different antiviral and RNA modification activities, which can alter the RNA modification characteristics observed in the viral RNA genome.
Among them, zinc finger antiviral protein (ZAP) is a key component of mammalian interferon-mediated immune response, its RNA binding domain and CpG II in viral RNA genome Nucleotides specifically bind. ZAP can inhibit viral replication and mediate viral genome degradation. Therefore, ZAP acts like an antiviral sentinel.
ZAP can not only fight against retroviruses like HIV-1, but also against Ecovirus type 7 and Zika virus. Strand RNA virus, similar to coronavirus. In particular, in ZAP-deficient cells, the selection for CpG in viral RNA disappeared. The paper mentions that this suggests that ZAP may be the only cytokine that targets CpG in the viral RNA genome.
In fact, many mammalian RNA viruses have evolved CpG defects. Previous studies have shown that in the presence of ZAP, the addition of CpG dinucleotides in these low-CpG viral genomes always results in virus replication and reduced virulence. This also prompted the development of vaccine development strategies involving increasing CpG to attenuate pathogenic RNA viruses.
The author quotes some previous thoughts that there is a correlation between the reduction of CpG in the viral RNA genome and the enhancement of toxicity. In RNA viruses, CpG decreases and virulence increasesThe strong connection is mainly due to the interferon-induced ZAP protein binding to the CpG dinucleotide in the viral RNA genome through its RNA-binding domain, inhibiting viral replication and promoting viral genome degradation.
Based on this, the author mentions that the reduction of ICpG in viral pathogens means an increased threat to public health, but the increase in ICpG reduces this threat because of this type of Viral pathogens, as ICpG increases and virulence decreases, will resemble natural vaccines. “In fact, many virus researchers have proposed to increase the CpG in the viral RNA genome to develop vaccines.”
In view of ZAP exhibiting tissue-specific expression, the infection is different Organized viruses may have different CpG signals, “This provides a way to identify the host tissue switch of the virus.”
The ICpG level of New Coronavirus and its closest RaTG13 is the lowest in its lineage
The ICpG level of New Coronavirus and its closest RaTG13 is the lowest in its lineage
In this study, Xia Xuhua tested the deposit in GenBank 1252 full-length genomes of beta coronaviruses. GenBank is a DNA sequence database established by the National Center for Biotechnology Information (NCBI) and contains DNA sequences from all available public sources.
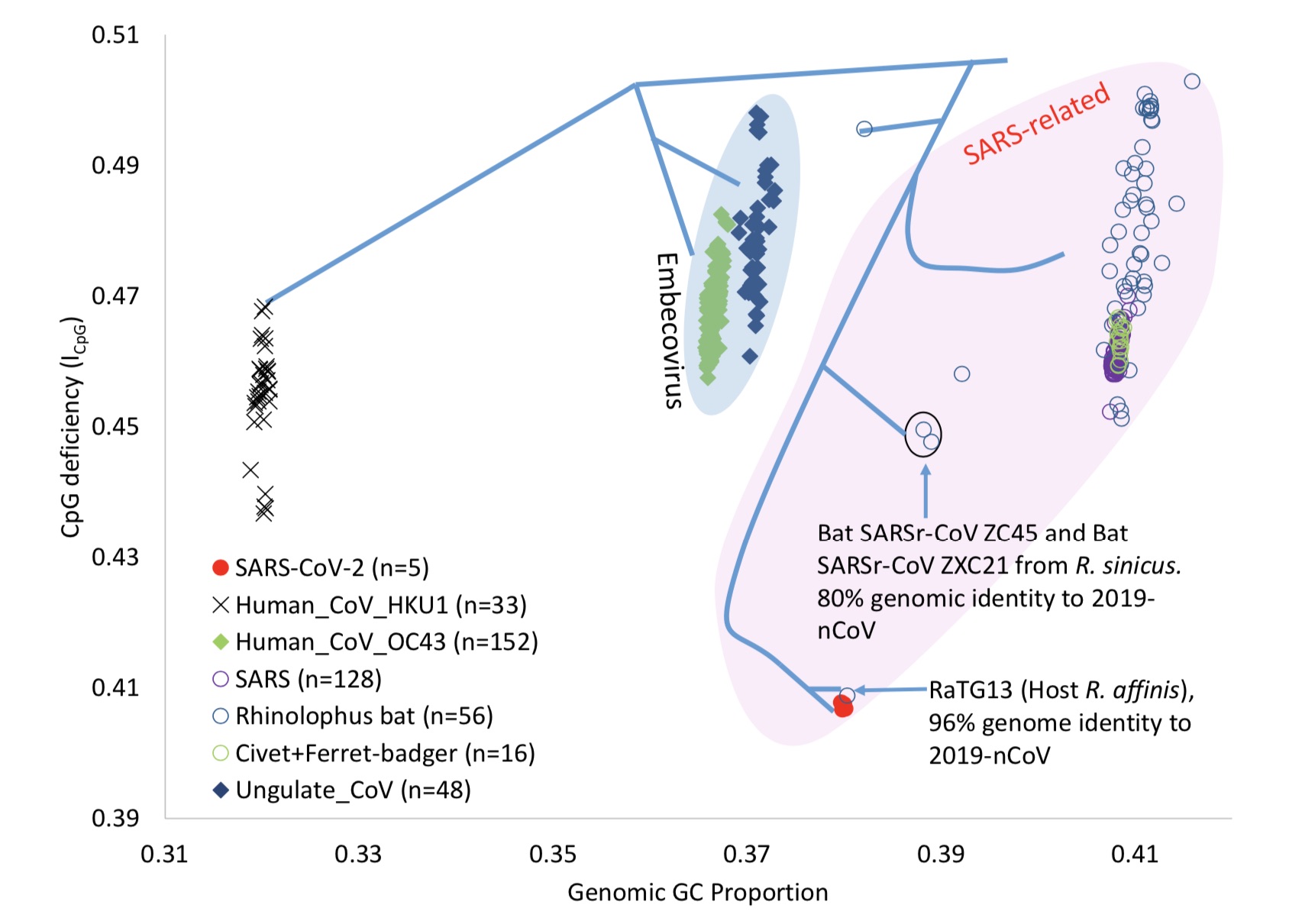 The results show that the ICpG level of New Coronavirus and its closest known close relative RaTG13 (researched by Shi Zhengli, a team of researchers from Wuhan Institute of Virology, Chinese Academy of Sciences, in Yunnan, China in 2013) is the lowest in its lineage, both It is the abnormal value of viral genome ICpG and GC%.
The results show that the ICpG level of New Coronavirus and its closest known close relative RaTG13 (researched by Shi Zhengli, a team of researchers from Wuhan Institute of Virology, Chinese Academy of Sciences, in Yunnan, China in 2013) is the lowest in its lineage, both It is the abnormal value of viral genome ICpG and GC%.
 The results show that the ICpG level of New Coronavirus and its closest known close relative RaTG13 (researched by Shi Zhengli, a team of researchers from Wuhan Institute of Virology, Chinese Academy of Sciences, in Yunnan, China in 2013) is the lowest in its lineage, both It is the abnormal value of viral genome ICpG and GC%.
The results show that the ICpG level of New Coronavirus and its closest known close relative RaTG13 (researched by Shi Zhengli, a team of researchers from Wuhan Institute of Virology, Chinese Academy of Sciences, in Yunnan, China in 2013) is the lowest in its lineage, both It is the abnormal value of viral genome ICpG and GC%.
The author said that it can be seen that the genome GC% and ICpG are present between different virus lineages of the same host, or between different hosts of the same virus lineage difference.
The author believes that the most striking thing is that the viral genome CpG in the RaTG13 lineage produced an isolated and sharp decline. After the outbreak, the team of Shi ZhengliStudies have shown that the sequence similarity between RaTG13 and New Coronavirus is 96.2%.
The author wrote in the paper: Unfortunately, RaTG13 was not sequenced in 2013, otherwise the decline of ICpG may be a warning because there are two very important meanings .
First of all, the virus may have evolved in a tissue with high ZAP expression. ZAP high expression is beneficial to the viral genome with low ICpG. Second, and more importantly, the survival of the virus indicates that it has successfully avoided ZAP-mediated antiviral defenses. “In other words, this virus has become sneaky and dangerous to humans.”
This test found that the ICpG value of the RaTG13 genome is 0.40875, Much lower than the ICpG values observed in all other β-coronavirus genomes collected from the bat species of Lysianthus. ICpG will fluctuate in the genomes of different virus lineages, but only RaTG13 ICpG has been observed to have a very low value.
The author mentions that this suggests that the ancestors of RaTG13 and the new coronavirus may have evolved in mammalian tissues with high expression of ZAP, resulting in abnormally low ICpG. This mammalian tissue may not be in the body of the chrysanthemum bat, because the ICpG content is not low in other bat families.
He believes that if a virus with a relatively low ICpG content can be identified, it means that the tissue and cellular environment of the candidate host species is very strong for CpG in the viral genome Selective.
Canine coronavirus: Is the new coronavirus derived from dogs?
Canine coronavirus: Is the new coronavirus derived from dogs?
As of February 3, 2020, among all beta coronaviruses provided by GenBank, there are 1127 unique genomes, of which 927 genomes have a clear host name. But the author mentions that, surprisingly, there is no available beta coronavirus genome in different natural hosts with a genome combination of ICpG and GC% similar to that of the new coronavirus and RaTG13.
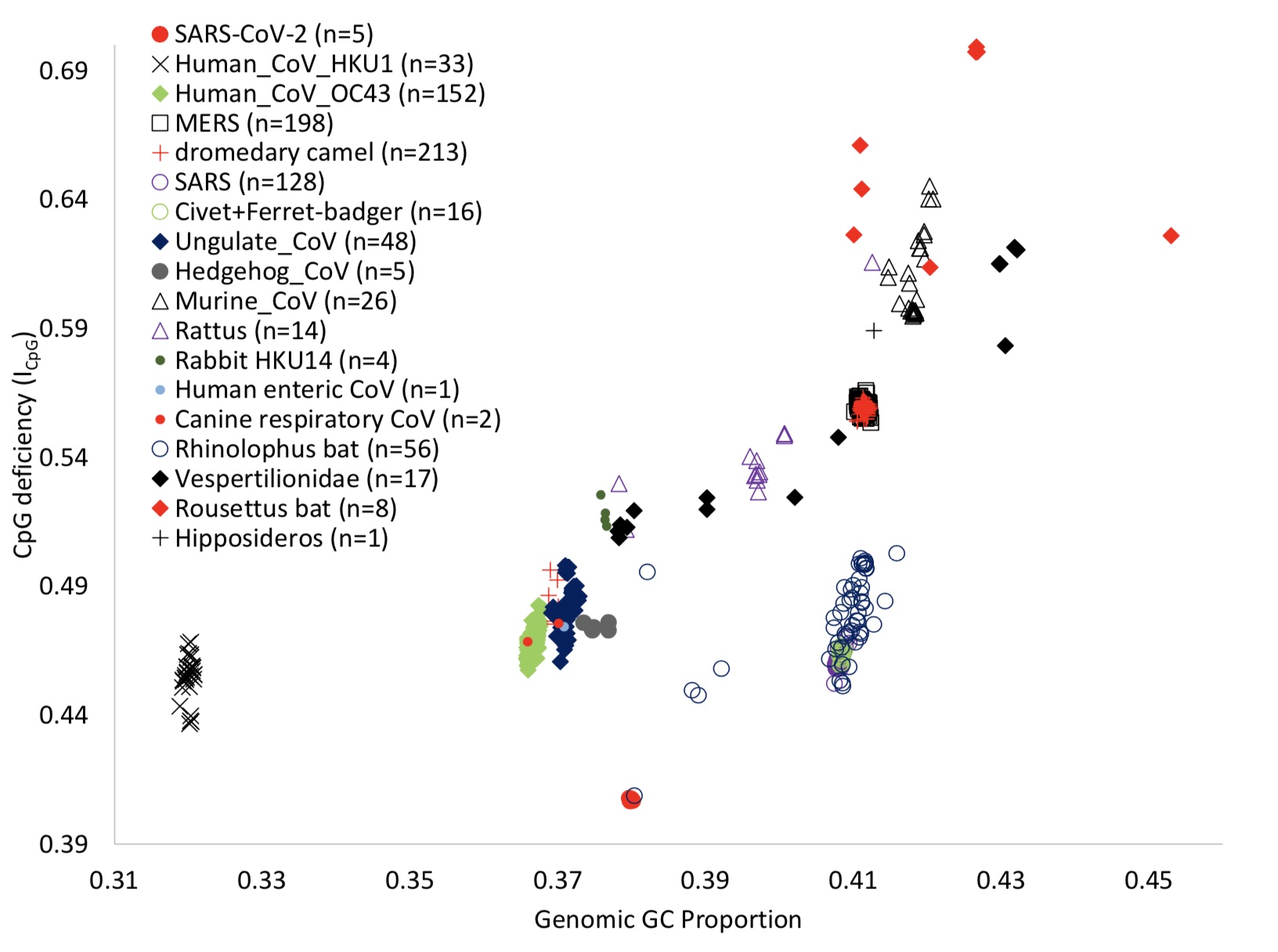 In order to find a mammalian host that might choose a virus lineage with a low ICpG value, the author expanded the search scope , Including all complete alpha coronavirus virus genomes in the study. Its genome testing range includes pets and dogs that humans are often exposed to, cattle, horses, sheep and pigs, as well as hedgehogs and mice, including giraffes and Africa Wild animals such as gazelle.
In order to find a mammalian host that might choose a virus lineage with a low ICpG value, the author expanded the search scope , Including all complete alpha coronavirus virus genomes in the study. Its genome testing range includes pets and dogs that humans are often exposed to, cattle, horses, sheep and pigs, as well as hedgehogs and mice, including giraffes and Africa Wild animals such as gazelle.
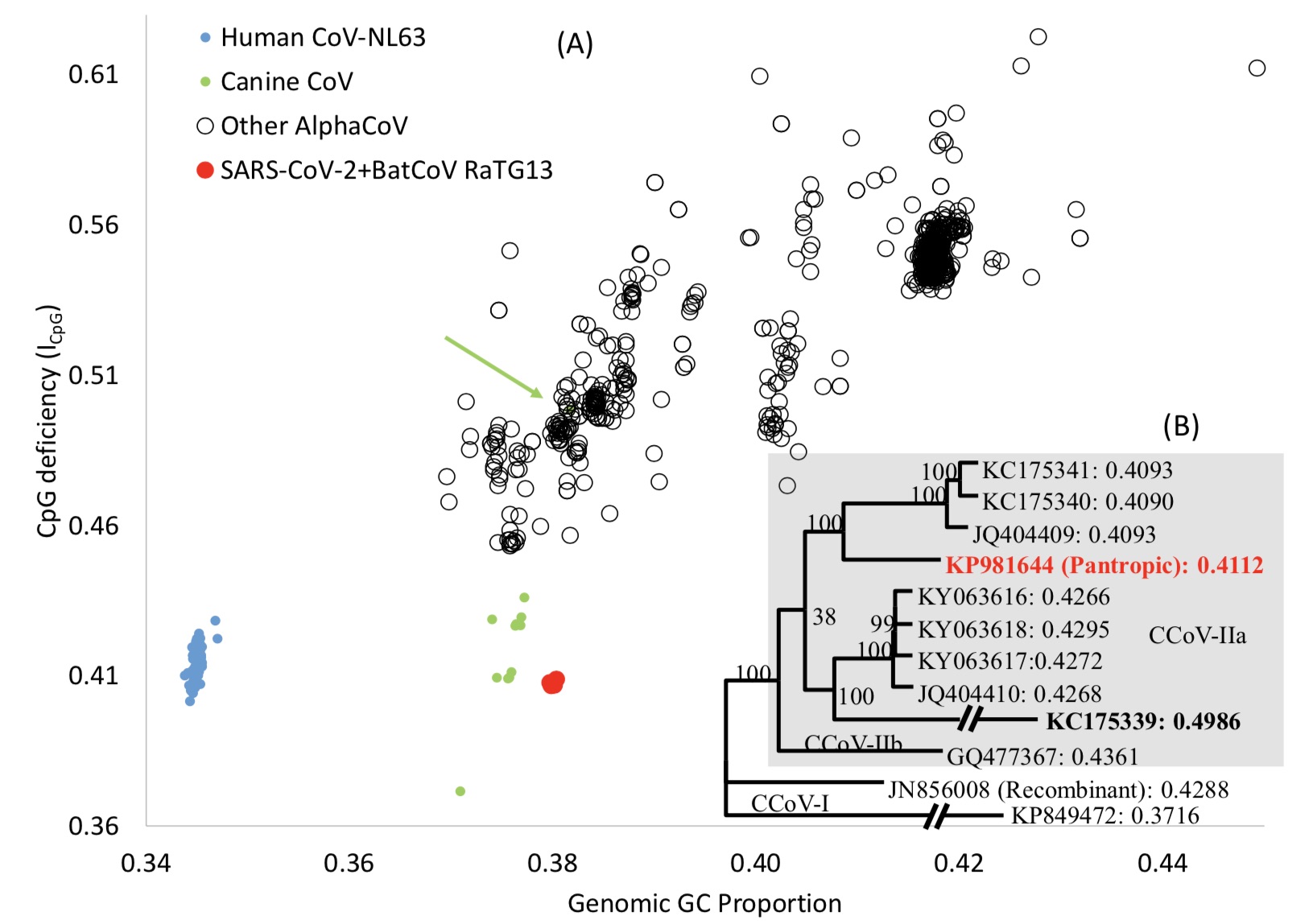 After expanding the search scope, the authors found that only the genomes from canine coronaviruses (CCoVs) have similar genome ICpG and GC% values to neocoronavirus and RaTG13. Canine coronaviruses cause highly infectious intestinal diseases in dogs worldwide, and the most deadly panthermovirus CCoV genome that invades multiple canine organs belongs to the branch with the lowest ICpG value observed.
After expanding the search scope, the authors found that only the genomes from canine coronaviruses (CCoVs) have similar genome ICpG and GC% values to neocoronavirus and RaTG13. Canine coronaviruses cause highly infectious intestinal diseases in dogs worldwide, and the most deadly panthermovirus CCoV genome that invades multiple canine organs belongs to the branch with the lowest ICpG value observed.
 In order to find a mammalian host that might choose a virus lineage with a low ICpG value, the author expanded the search scope , Including all complete alpha coronavirus virus genomes in the study. Its genome testing range includes pets and dogs that humans are often exposed to, cattle, horses, sheep and pigs, as well as hedgehogs and mice, including giraffes and Africa Wild animals such as gazelle.
In order to find a mammalian host that might choose a virus lineage with a low ICpG value, the author expanded the search scope , Including all complete alpha coronavirus virus genomes in the study. Its genome testing range includes pets and dogs that humans are often exposed to, cattle, horses, sheep and pigs, as well as hedgehogs and mice, including giraffes and Africa Wild animals such as gazelle.
 After expanding the search scope, the authors found that only the genomes from canine coronaviruses (CCoVs) have similar genome ICpG and GC% values to neocoronavirus and RaTG13. Canine coronaviruses cause highly infectious intestinal diseases in dogs worldwide, and the most deadly panthermovirus CCoV genome that invades multiple canine organs belongs to the branch with the lowest ICpG value observed.
After expanding the search scope, the authors found that only the genomes from canine coronaviruses (CCoVs) have similar genome ICpG and GC% values to neocoronavirus and RaTG13. Canine coronaviruses cause highly infectious intestinal diseases in dogs worldwide, and the most deadly panthermovirus CCoV genome that invades multiple canine organs belongs to the branch with the lowest ICpG value observed.
The author believes that it is important to emphasize that canines, like camels, also have coronaviruses that infect their respiratory systems. CRCoV has two genomic sequences, and their genomic ICpG values are 0.4756 and 0.4684, which are significantly higher than CCoVs that infect the digestive system (Figure 3A). Therefore, similar to the camel-infected coronavirus, the ICpG content of CCoVs infected with the digestive system of dogs is much lower than that of CRCoVs infected with the respiratory system of dogs.
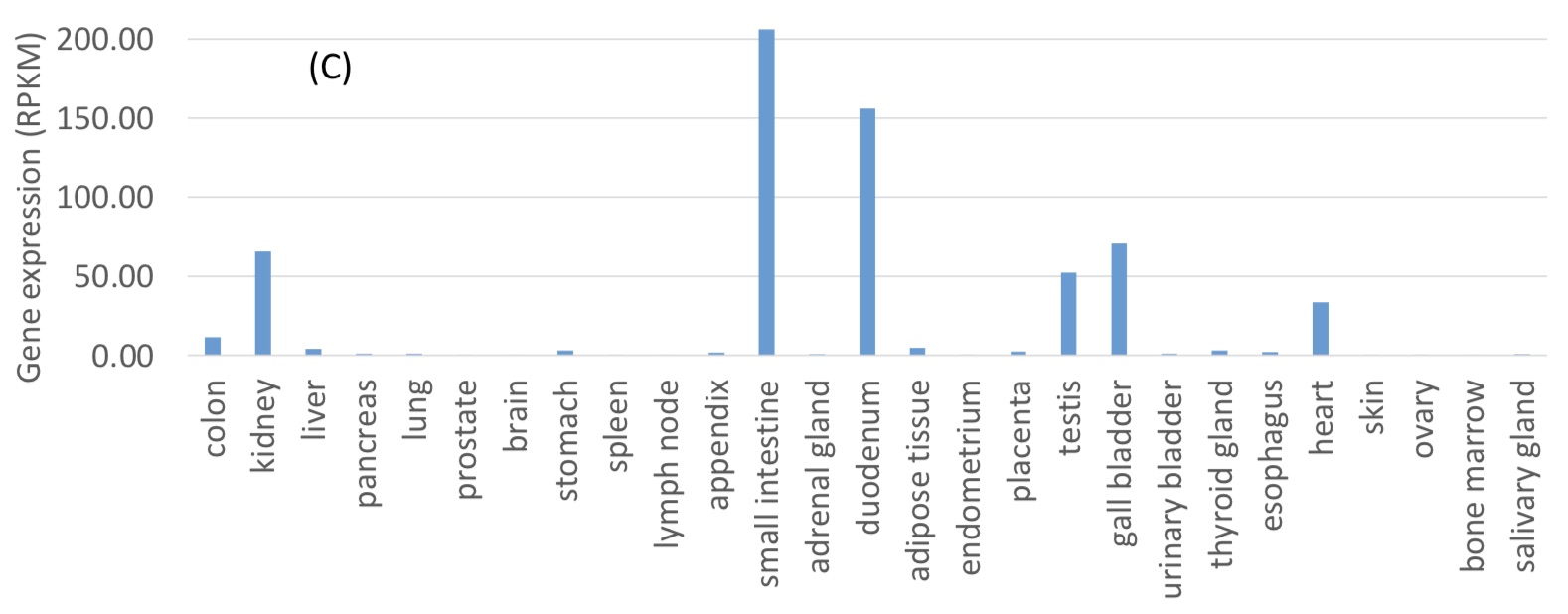 Current research believes that the cell receptor of the new coronavirus entering the cell is ACE2 (angiotensin converting enzyme 2)
Current research believes that the cell receptor of the new coronavirus entering the cell is ACE2 (angiotensin converting enzyme 2)
 Current research believes that the cell receptor of the new coronavirus entering the cell is ACE2 (angiotensin converting enzyme 2)
Current research believes that the cell receptor of the new coronavirus entering the cell is ACE2 (angiotensin converting enzyme 2)
. ACE2 is widely expressed in the human digestive system, with the highest expression levels in the small intestine and duodenum, and relatively low expression in the lungs. This indicates that the mammalian digestive system is likely to be infected by coronavirus.
The author believes that a recent report further confirms this explanation that most patients with COVID-19 also have digestive discomfort.
The author concludes in the paper that these observations provide hypotheses for the origin and initial spread of the new coronavirus. First, the ancestors of the new coronavirus and bat-derived coronavirus RaTG13 infected the intestinal tract of a mammal (such as bat meat eaten by canines or humans). Second, the strong selectivity of canine enterovirus RNA genome for CpG may lead to the rapid evolution of the virus. A large number of mutations from CpG to UpG lead to a reduction in the genome ICpG and GC%. Third, dogs lick the anal area during mating and other conditions, helping the virus spread from the digestive system to the respiratory system. Finally, the reduced viral genome ICpG allows the virus to evade the human ZAP-mediated immune response and become a serious human pathogen.
He emphasized that this shows the importance of monitoring SARS-like coronavirus in wild dogs in the fight against the new coronavirus.
Academic questioning
Academic questioning
However, although the paper was officially published after peer review, it has attracted many questions. The doubters generally believe that this is just a hypothesis, based on computer analysis of various coronavirus genomes, a theory that has not been proven by any established facts. Professor James Wood, director of the Department of Veterinary Medicine at the University of Cambridge in the United Kingdom, said, “I don’t think any dog owners will be worried about this research.” He believes that there is nothing in this paper that supports this hypothesis. Too many inferences and too few direct data. “
William Schaffner, professor of preventive medicine and infectious diseases and infectious disease expert at Vanderbilt University School of Medicine , “Should we worry about getting the virus from our dog, or should we worry about transmitting the virus to our dog?” He believes that the answer to both questions is no.
“This study was based on the reanalysis of old data without any new data.” Ben Neuman, director of the Department of Biological Sciences at Texas A & M University Added that this study is similar to a previous paper that mistakenly considered snakes as the origin of the new coronavirus. “Need some new data to finally solve the mystery of the origin of the new coronavirus, the conclusion that cats or dogs are the intermediate host of the new coronavirus is highSpeculative, should not be presented as facts. ”
Similarly, Pleuni Pennings, an expert in ecology and chemistry from San Francisco State University in the United States, also believes that some of the data and conclusions in this paper by Xia Xuhua cannot be established. The team of Pennings Many viruses have been tested for CpG levels, and she believes that the logic of this research is weak.
Pennings investigated in a study published in the journal PLOS Genetics in 2018 Studied the CpG level of the HIV virus and studied the evolution of this pathogen in the human body. She also led a similar study to study several other viruses, including dengue virus, influenza virus, hepatitis B and C viruses, understand The frequency with which these viruses lose or acquire CpG sites through mutations.
Pennings says that generating CpG mutations can be costly for viruses because they alert the body to infection, So over time, evolutionary forces will minimize them. “There are many viruses with lower CpG values than new coronaviruses,” she said. “When you look at all the viruses, the CpG value is a little bit Not surprisingly. ”
She added that this research by Xia Xuhua did find that the new coronavirus contains fewer CpG sites than coronaviruses transmitted by other animals, and assumes this discovery Yes, then this raises the question of why this happens. Pennings said that even if there are evolutionary reasons that can explain why the new coronavirus has lost the CpG site, the evolutionary reasons cannot make the virus infect Humans have special advantages.
pointed out in a paper against Xia Xuhua, “Studies have shown a link between the reduction of CpG in viral RNA genomes and the enhancement of toxicity,” which means The low CpG virus seems to be related to more serious infections. However, Pennings reminds that viruses with a small number of CpG sites must be more deadly. She cited, for example, that the BK virus contains few CpG sites, about 60% to 80% of adulthood. This virus is found in human kidneys, but it usually only causes symptoms in immunosuppressed people.
It ’s also worth noting that there are currently Some studies also It does not support dogs playing an important role in this epidemic. Prior to April 8, local time, the top academic journal “Science” published online the academician of the Chinese Academy of Sciences and the Harbin Veterinary Institute of Chinese Academy of Agricultural Sciences Chief Science of the Innovation Team for Prevention and Control ResearchChen Hualan, director of the Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences, and Bu Zhigao, director of the National Animal Disease Prevention and Control High-level Biosafety Laboratory A series of new coronavirus infection tests found that the new coronavirus does not replicate well in dogs, pigs, chickens and ducks, but it is very effective in ferrets and cats. They also found that the virus can spread between cats through respiratory droplets.
In fact, since the New Crown epidemic, some experimental teams engaged in virus tracing have successively included snakes, pangolins, cats and other animals into the list of suspected intermediate hosts of the new crown virus. However, as of now, there is still no convincing conclusion.