So far, the origin of the new coronavirus is still a mystery to the intermediate host, and its ability to infect pets and livestock is still unclear. Recently, the Ministry of Education’s “Laboratory Health and Food Safety” International Cooperation Joint Laboratory and other teams have discovered that ACE2 (angiotensin converting enzyme 2) of species known to be infected with new coronavirus can tolerate many amino acid changes, indicates that The barrier of species may be low, that is, the risk of cross-species transmission is greater. At the same time, the new coronavirus may not have specifically adapted to any of its putative intermediate hosts.
The team analyzed the X-ray structure of the newly released human ACE2 binding to the S protein receptor binding domain (RBD) of the new coronavirus (SARS-CoV-2), and then Predict the binding of ACE2 of different pets, farm animals, putative intermediate hosts of viruses, etc. to viral proteins.
Effectively binding to the host receptor ACE2 is the primary prerequisite for cells infected by the new coronavirus, which determines the range of viral hosts. Researchers have found that compared to human ACE2, the ACE2 of bats, civet cats, etc. have undergone a lot of amino acid changes, but they are still able to bind to the new coronavirus S protein , of which the bat ACE2 protein contains at least 5 amino acid changes .
Research indicates that pets (cats) and domestic animals are at risk of being infected by SARS-CoV-2 because they are at the ACE2-S protein interface compared to other animals The amino acid changes are less.
At the same time, pangolin ACE2 exhibited 7 changes relative to human ACE2, and there were similar numbers of substitutions in ACE2 of bats, raccoons, and civet cats, indicating that SARS-CoV- 2 It may not be particularly suitable for a certain intermediate host ACE2, that is, the mechanism of neocoronavirus infection of animals may be different from that of humans.
These analyses provide new insights into the SARS-COV-2 invasion receptor and its animal origin and natural origin. The research results have been published online in the biomedical preprint platform bioRxiv recently, entitled “Comparison of SARS-CoV-2 spike protein binding to human, pet, farm animals, and putative intermediate hosts ACE2 and ACE2 receptors”.
The research team includes “Animal Health and Food Safety”, Ministry of Education, Nanjing Agricultural UniversityThe International Cooperation Joint Laboratory, the Institute of Virology, Veterinary College of Free University of Berlin, Germany, and the Changchun Veterinary Research Institute of the Chinese Academy of Agricultural Sciences, etc. .
ACE2, such as bats and civet cats, can tolerate a large number of amino acid changes and still bind to the new crown S protein
ACE2, such as bats and civet cats, can tolerate a large number of amino acid changes and still bind to the new crown S protein
Studies indicate that SARS-CoV-2 may have originated For bats, pangolins may be potential intermediate hosts. Specifically, SARS-CoV-2 has a high degree of nucleotide sequence identity with the bat coronavirus RaTG13, but the gene encoding the viral S protein in the middle of its genome may have been recombined with the pangolin-like coronavirus.
The S protein of the new coronavirus is responsible for binding to the receptor to invade the host. During the infection process, the S protein is cleaved by the host protease into the S1 subunit and S2 subunit, respectively. Conduct receptor binding and membrane fusion. Among them, S1 contains a receptor binding domain (RBD), which is crucial in determining tissue orientation and host range.
When the virus invades the human body, RBD binds to the human receptor ACE2 (angiotensin converting enzyme 2). ACE2 is a human receptor membrane protein shared by New Coronavirus and SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus), has homology with human angiotensin converting enzyme 1 and is shown in the human cardiovascular system and many other organs Out protection.
Previous studies have shown that SARS-CoV-2 has poor replication ability in dogs and pigs, but cats may be infected. Using comparative bioinformatics and structural methods, the researchers analyzed the X-ray structure of human ACE2 and the viral S protein structure that bind to the S protein receptor binding domain of SARS-CoV-2.
Due to the different transfection of the Hela cell line containing the ACE2 receptor, ACE2 of humans, pigs, civet cats and bats are all resistant to SARS-CoV -2 susceptible, but ACE2 in mice is not sensitive.
In order to evaluate which interacting amino acids in ACE2 are essential for its binding to viral S protein, the researchers conducted human ACE2 sequences with other species ’ACE2 sequences Comparison to determine the amino acid sites that play a key role in the binding of the virus to ACE2.
Studies show that when the neocoronavirus invades, 17 residues in its S protein are in contact with 20 amino acids in ACE2, of which 8 amino acids are in contact with 13 residues form hydrogen bonds.
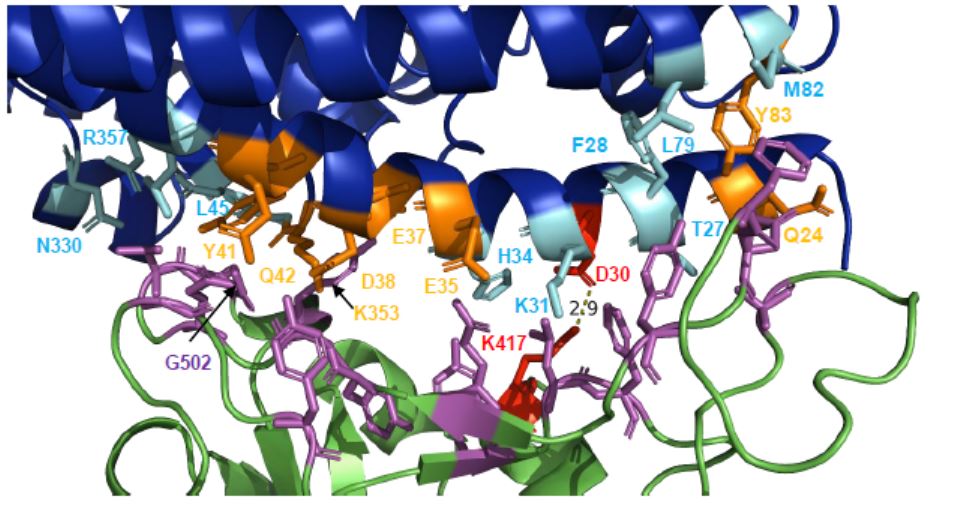
Interaction surface of S protein (green) and human ACE2 (blue) of SARS-CoV-2

Interaction surface of S protein (green) and human ACE2 (blue) of SARS-CoV-2
According to previous research, the S protein of SARS-CoV-2 has a higher affinity for its receptor than the S protein of SARS-CoV. The amino acids in SARS-CoV-2 form more hydrogen bonds than when interacting with ACE2.
Researchers found that compared to human ACE2, porcine ACE2 has 5 amino acid substitutions on the surface interacting with the S protein of SARS-CoV-2, of which Three are located on the periphery of the binding site.
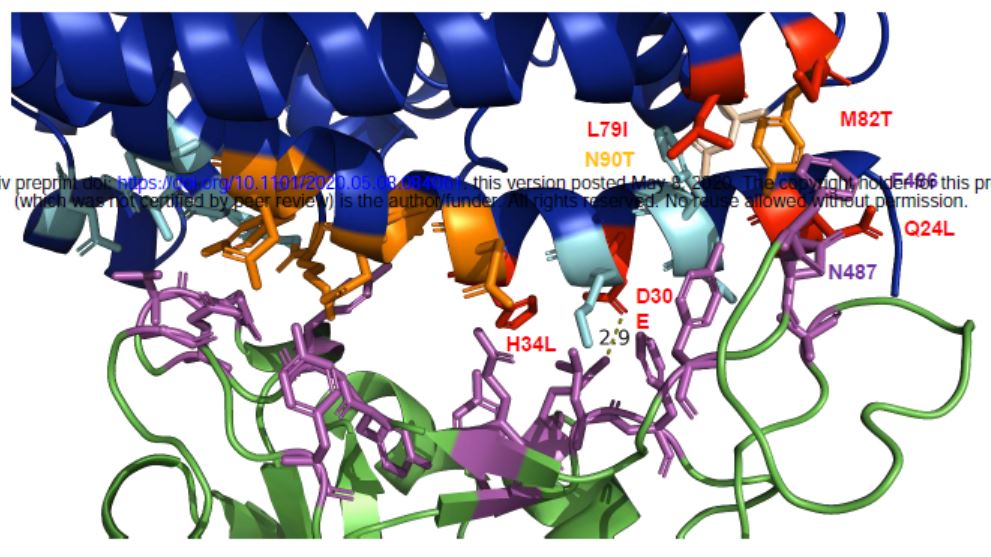
Compared with human ACE2, ACE2 in pigs has amino acid substitutions on the surface interacting with the S protein of SARS-CoV-2, 5 substitution The location is marked in red.

Compared with human ACE2, ACE2 in pigs has amino acid substitutions on the surface interacting with the S protein of SARS-CoV-2, 5 substitution The location is marked in red.
Researchers extracted three sets of amino acid sequences from the database of ACE2 gene of Chinese rhinoceros, which are from Guangxi, Hubei and Yunnan. Surprisingly, despite their high overall amino acid identity (99%), they showed a largerdifference.
Researchers found that the bat ACE2 protein contains at least 5 amino acid changes compared to human ACE2, three of which are substituted in all bat ACE2 sequences and have The same residues in porcine ACE2, but replaced by other amino acids. The team believes that bats are widely regarded as the natural host of the new coronavirus. It is surprising that so many changes in the ACE2 gene have occurred between different areas of the Chinese chrysanthemum, and further research is needed. However, a reason that is easier to speculate is that local co-evolution between bats and new coronaviruses will drive these amino acid changes.
Next, the researchers analyzed ACE2 in ling cats, which has been shown to be a receptor for SARS-CoV-2. Compared with human ACE2, the ACE2 of the smart cat has 7 amino acid changes, three of which are the same as the ACE2 in pigs.
Compared with pigs, bats, and civet cats, mice that do not support SARS-CoV-2 cell infection interact with SARS-CoV-2 S protein There are 8 amino acid substitutions on the surface.
In short, compared with human ACE2, a large number of amino acid changes in the interaction surface of and the new coronavirus S protein do not affect its binding. u>, in which there are 5 amino acid substitutions in pig ACE2, and 7 amino acid changes in smart cat ACE2.
Even if bat ACE2 obtains new and important N-glycosylation sites during multiple amino acid substitutions, it will not prevent SARS-CoV-2 from bat ACE2 Used as a receptor in transfected cells.
Can the new crown cross species barriers? The risk of cross-species transmission is high and needs continuous monitoring.
Can the new crown cross species barriers? The risk of cross-species transmission is high and needs continuous monitoring.
Prior to April 3, the team of Professor Jin Meilin of Huazhong Agricultural University and Professor Shi Zhengli of Wuhan Institute of Virology, Chinese Academy of Sciences, etc A research paper published on bioRxiv stated that the serum ELISA test of 102 cats showed that sera from 15 cats (14.7%) were positive for the receptor binding domain (RBD) of neocoronavirus. But so far, there is no evidence that cats can infect people.
In this study, researchers also explored the species barriers (cross-species) in pets when the new coronavirus S protein binds to the receptor ACE2Species). Compared with humans, the amino acids of dog ACE2 in contact with viral S protein contain 5 amino acid changes, 3 of which are also replaced in ACE2 of pigs.
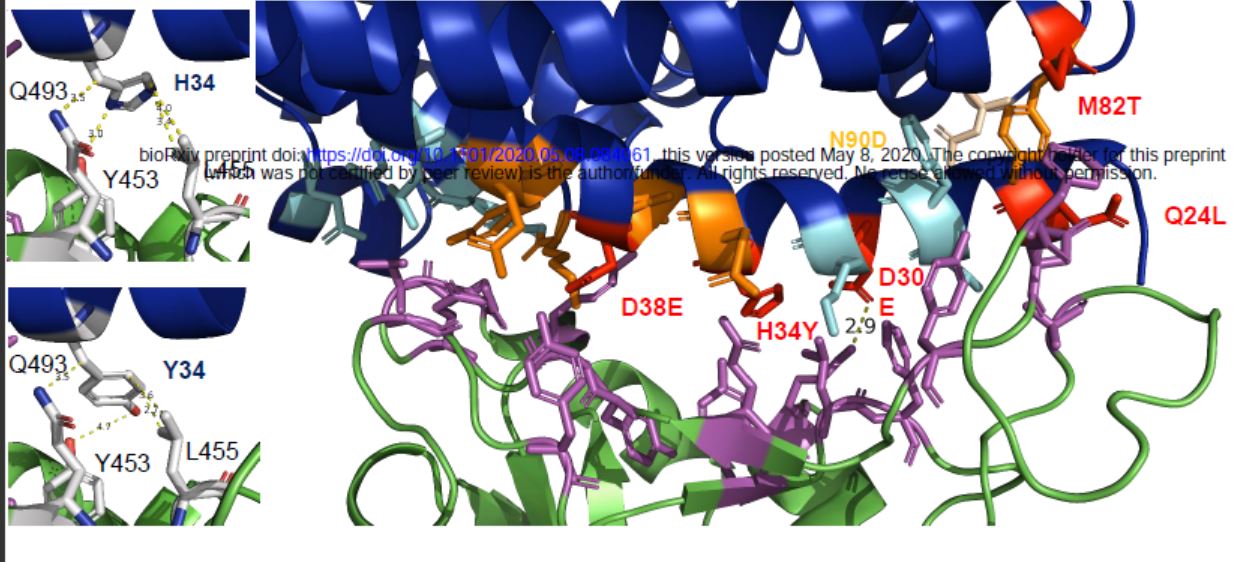
Amino acid changes when dog ACE2 is in contact with viral S protein

Amino acid changes when dog ACE2 is in contact with viral S protein
and Compared to humans, cat ACE2 has only 4 amino acid changes, and they are also present in dog ACE2. The researchers previously analyzed the ability of ACE2 from pigs, dogs and cats to bind to the viral S protein. The results showed that SARS-CoV-2 has a weak replication ability in dogs, but it can effectively replicate in cats and fly through the Mo spread to other cats.
It was previously reported that the tigers and lions of the Bronx Zoo in New York City were infected with SARS-CoV-2. So the researchers analyzed their ACE2 gene. In ACE2 of cats and tigers, the researchers detected a 1 amino acid difference, but the residues they contacted with the viral S protein were the same, which explains why tigers are also susceptible to SARS-CoV-2 infection.
Compared with cat ACE2, there is another conservative change in lion ACE2, and like dog ACE2, one of its N-glycosylation sites is missing.
The researchers next analyzed several animal models that showed a higher risk of spreading the new crown. Recent studies have shown that ferrets are more sensitive to SARS-CoV-2 infection, and although they are less efficient, they can spread the virus through droplets. The results of the study showed that the ferret ACE2 showed exactly the same 5 amino acid changes as the dog ACE2.
In addition, the Syrian hamster (Syrian hamster) ACE2 protein contains only two amino acid substitutions compared to human ACE2; as an animal model of higher susceptibility to new coronavirus, As a pet in some families, guinea pig ACE2 has 7 amino acid substitutions.
In farm animals, chicken ACE2 protein contains 10 amino acid changes, and a N-glycosylation site is lost at the position marked 90. Duck ACE2 also has 10 amino acid substitutions, Studies suggest that the lack of sensitivity of chickens and ducks to experimental SARS-CoV-2 seems to be due to the virus is unable to bind the ACE2 receptor of birds .
Compared with humans, ACE2 of cattle and sheep has only two amino acid changes , and it retains the N-glycosylation site at the 90 position of human ACE2. Therefore, the ACE2 proteins of both species may play the role of SARS-CoV-2 receptors, and it is likely to be a highly susceptible animal model. The researchers pointed out that it is necessary to strengthen monitoring to show whether they are CoV-2 is susceptible.
Compared with humans, ACE2 of cattle and sheep has only two amino acid changes , and it retains the N-glycosylation site at the 90 position of human ACE2. Therefore, the ACE2 proteins of both species may play the role of SARS-CoV-2 receptors, and it is likely to be a highly susceptible animal model. The researchers pointed out that it is necessary to strengthen monitoring to show whether they are CoV-2 is susceptible.
Overall, Almost all mammalian species (cats and ferrets) known to be susceptible to SARS-CoV-2 infection have many of their ACE2 proteins There are mutations in amino acids . This indicates that these species, especially those in close contact with humans, are at risk of virus infection. SARS-CoV-2 may establish an additional virus bank in one of the animals.
Researchers also found that pigs will not be infected with new coronavirus and dogs will not be effectively infected. The binding site of ACE2 of these two animals is only There are small changes, but their ACE2 levels in the respiratory tract are relatively low.
This also shows that the binding of the viral S protein to the ACE2 receptor is only the first step of virus invasion, and the level of ACE2 in different tissues of the same species may also be in the virus It plays an important role in transmission, for example, the expression of ACE2 is lower in the upper respiratory tract of certain pets and livestock. Therefore, the research team believes that the risk of neocoronavirus animal infection needs continuous monitoring to explore the source of animal infection and the potential risk of cross-species transmission.