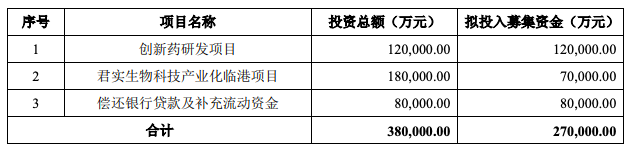
It is planned to invest 1.2 billion yuan to raise funds in innovative drug research and development projects.
On June 22, Junshi Bio (01877.HK/688180.SH) issued an initial public offering of shares and a prospectus for listing on the Science and Technology Board.
According to the prospectus, Junshi Biology plans to issue 87.13 million A shares to be listed on the Shanghai Science and Technology Board. The number of publicly issued shares accounts for 10% of the total share capital after this issuance. %, a preliminary inquiry will be made on June 29 to determine the A-share issue price. CICC is the main underwriter, and Guotai Junan and Haitong Securities are the joint underwriters.
The funds raised from the listing of the Science and Technology Board will be used for R&D expenditures, and it is planned to invest 1.2 billion yuan to raise funds for innovative drug research and development projects. Of the remaining funds, 7 100 million yuan is used for Junshi Biotech Industrialization’s Lingang project, 800 million yuan is used to repay bank loans and supplement working capital.
The picture is from Junshi Biological Prospectus
R&D expenses have always been the bulk of Junshi’s expenditure. In 2017, 2018 and 2019, their R&D expenses amounted to 275 million yuan, 538 million yuan and 946 million yuan, respectively. In the first quarter, Junshi Biology’s R&D expenses rose again, up 11.9% year-on-year to 217 million yuan.
Since the establishment of Junshi Biological Company, it has focused on drug research and development, and has more research and development pipelines, and several research projects are entering phase II and III clinical trials. Therefore, the company has incurred large research and development expenses for several consecutive years. .
However, in comparison, Jun Shi Biological’s financial situation is improving.
The prospectus shows that in the first quarter of 2020, Jun ShishengThe company realized revenue of 172 million yuan, an increase of 117.7% year-on-year. The net loss attributable to the parent company owner was 2.29 yuan, a year-on-year loss of 38.11%, and a loss of 371 million yuan in the same period last year.
The increase in revenue was mainly due to the increase in drug sales revenue during the reporting period. Triplezumab (recombinant humanized anti-PD-1 monoclonal antibody injection, trade name: Tuo Yi) developed by Junshi Bio was officially approved by NMPA (National Drug Administration) with conditions in December 2018 , Starting sales at the end of February 2019.
Junshi Bio’s 2019 annual report released on the Hong Kong Stock Exchange showed that the company’s revenue for the year was 775 million yuan, and Tuoyi’s sales revenue accounted for 99.9% of its total revenue.
However, because the company has a large number of R&D pipelines, with the advancement of R&D projects, including the extension of indications for the extension of listed products, as well as the progress of some clinical projects and the development of overseas clinics, R&D expenditures are relatively large For other reasons, Junshi Biology expects its losses from January to June 2020 will be further expanded.
Junshi Biological estimates that the net loss attributable to shareholders of the parent company will be 563 million to 681 million yuan in the first half of 2020, an increase of 93.46% to 133.97% year-on-year; in the first half of the year, it will realize revenue of 456 million to 561 million yuan, a year-on-year An increase of 47.34% to 81.28%.
As of May 17, 2020, Junshi Biological has a total of 21 products in research, 13 are original innovative drugs independently developed by the company, and 8 are jointly developed with partners. In addition to the top benefits that have already been put on the market, another 9 products have been approved by IND and 12 products are in the preclinical research stage.