Scientists are still cautious about when the new crown vaccine will be launched in early 2020. This is not surprising, because the fastest vaccine launch rate in human history also took 4 years.
The previous record for the fastest vaccine was relatively remote, and it was in the 1960s. It took 4 years for scientists to obtain the mumps virus strain to the final vaccine approval. Therefore, when the new crown epidemic broke out, the scientific community’s most optimistic forecast for the approval of the new crown vaccine was also the summer of 2021.
However, as of early December 2020, several vaccines have received more protection data than expected in large trials. On December 2, the vaccine produced by the pharmaceutical giant Pfizer and the German biotech company BioNTech became the first COVID-19 vaccine approved for emergency use in the United States.
Regarding the “lightning speed” of the new crown vaccine development, a report published in Nature magazine recently analyzed that this may profoundly change the future of vaccine science.
Natalie Dean, a biostatistician at the University of Florida, said in an interview with Nature that this speed of development “challs the possibility of us developing a vaccine The whole paradigm of “. Scientists hope that similar time can be used to produce other vaccines. This is of great practical significance, because diseases such as malaria, tuberculosis, and pneumonia cause millions of deaths worldwide every year. Scientists predict that there will be more deadly pandemics in the future.
The research and development of global vaccine lightning speed cannot be separated from the efforts of Chinese researchers. Gao Fu, academician of the Chinese Academy of Sciences and director of the Chinese Center for Disease Control and Prevention, said in an interview with Xinhua News Agency on December 29, “First of all, we in China have made the isolated viruses and sequencing results transparent to the world, and soon entered the research and development of the new crown pneumonia vaccine. “
The basis of mRNA vaccines is the viral gene sequencing data. A few hours after Chinese researchers released the genome sequence of the new coronavirus for the first time globally, the vaccine development work funded by the Epidemic Defense Innovation Alliance (CEPI) began in full swing. According to a previous report in Science, Barney Graham, deputy director of the NIAID Vaccine Research Center, began analyzing the genome sequence with his team on January 11, local time. On the following January 13, Graham discussed his findings with Moderna’s researchers, and on January 14, they signed a cooperation agreement. 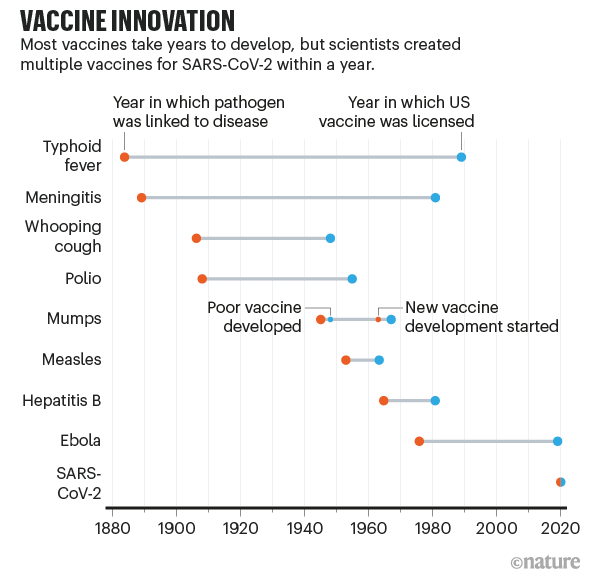 mRNA technology matures
mRNA technology matures
Dan Barouch, director of the Harvard Medical School’s Virology and Vaccine Research Center, said that the development experience of the new crown vaccine will definitely change the future of vaccine science. He said, “This shows that in an emergency that truly threatens the world, if there are sufficient resources, vaccine development can proceed quickly.”
Barush is accepting the ” Nature said in an interview that new methods for producing vaccines, such as messenger RNA (mRNA) technology, have been validated. This shows that vaccine development can be greatly accelerated without compromising safety.
According to the analysis of Nature, there are some other favorable conditions behind the rapid development of the new crown vaccine around the world. First of all, the scientific community has done more research on related coronaviruses. In addition, mRNA production of vaccines is also a faster technology. In addition, the huge capital investment allows major companies to conduct multiple trials in parallel, and the approval of the regulatory agency is faster than normal. Some of these factors may translate into other vaccine jobs, especially faster vaccine production technology platforms.
However, if the next vaccine development is to achieve success similar to the new crown vaccine, first a large amount of research and development funds will be needed. This is only possible under similar social and political urgency. appear. In addition, it depends on the virus itself. The new coronavirus mutates relatively slowly, and humans are relatively familiar with the coronavirus family.
In an interview with Xinhua News Agency, Gao Fu stated that the mRNA vaccine route is a vaccine developed for cancer patients and used to treat cancer. “For patients and healthy people. The use is different. I don’t say whether it will have side effects in the future, but at least it has not been ruled out, because humans will be the first time to use mRNA vaccines on healthy people, so there is a safety issue behind it. As a professional Personnel, we must analyze good or bad, this is a scientific attitude.”
Gao Fu said, “For now, the layout of inactivated vaccines was very good. Yes, and we are walking very fast, and the feedback in some places is also very effective.”
Research from many years ago
Strictly speaking, the research of scientists on the new crown vaccine did not start in January 2020. Researchers have been paying attention to coronaviruses for many years. Certain coronaviruses in this family can cause serious diseases, such as SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome). In response to these coronaviruses, scientists not only research and develop new vaccines against these coronaviruses, these efforts have not been in vain.
Conventional vaccines contain viral proteins or inactivated forms of the virus, which can stimulate the immune system of the vaccinated and thus can resist infection by live viruses. However, the two new crown vaccines announced in large-scale clinical trials (phase) only used a series of mRNA in the lipid coat. This mRNA encodes the key protein of SARS-CoV-2. Once the mRNA enters the human cell, the body produces this protein. That is the antigen-the foreign molecule that triggers the immune response. Vaccines produced by Pfizer, BioNTech, and Moderna, a US pharmaceutical company, use mRNA encoding a spike protein that docks with human cell membrane receptors, allowing coronaviruses to invade cells.
Iwasaki (Akaki Iwasaki), an immunologist at Yale School of Medicine, said that scientists have invested a lot of research on the mRNA vaccine technology platform. Iwasaki has been committed to nucleic acid vaccine research for more than two decades. She said that basic research on DNA vaccines began at least 25 years ago, while the mRNA vaccine platform has been conducting high-intensity research for 10 to 15 years, some of which are aimed at developing cancer vaccines. Today, mRNA technology has matured day by day, but the technology was not ready 5 years ago.
The MERS and SARS research of the National Institute of Allergy and Infectious Diseases (NIAID) plays an important role in promoting mRNA technology. Their researchers found that it is best to adjust the RNA sequence before the viral spike protein is docked with the host cell receptor, so that a stable form of the spike protein can be obtained. Barney Graham, deputy director of the NIAID Vaccine Research Center, said that if the original state of the virus before fusion can be captured, better vaccine antigens can be obtained. This work enabled the Moderna team that worked with NIAID to take the lead after obtaining SARS-CoV-2 sequencing in January. Dean, a biostatistician at the University of Florida, said that people have been paying close attention to the coronavirus, which has accelerated the entire vaccine development process.
AstraZeneca and Oxford, UKThe vaccine jointly produced by the university showed efficacy in a phase III clinical trial in November. The vaccine does not use mRNA technology. In contrast, the viral vector used in the vaccine has genetic material that encodes the SARS-CoV-2 spike protein. The AstraZeneca team chose a modified form of adenovirus isolated from chimpanzee feces. Beate Kampmann, director of the London School of Hygiene and Tropical Medicine’s Vaccine Center, said that such conventional vaccines have also made great progress, which also comes from previous studies on SARS, MERS, Ebola and malaria. In addition, conventional vaccines using this technology are currently cheaper than mRNA vaccines.
Iwasaki said that vaccine researchers are thankful in many ways. She said that the mutation of the new coronavirus is not very fast, and it does not have an effective strategy to defeat the human immune system like HIV. In this respect, it is even inferior to the herpes virus and influenza virus. In contrast, herpes virus has a greater immune escape ability, it can actively prevent antibody binding, which makes it more difficult to find effective drugs against it. The rapid mutation of influenza virus requires the public health department to use different vaccine preparations in each influenza season.
Increased investment in vaccines
The slowest part of vaccine development is not to find candidate vaccines, but to test them. This usually takes several years. The company conducts efficacy and safety tests on animals first, and then tests on humans. Human trials require three stages, the number of people will gradually increase, and the cost will increase accordingly. The new crown vaccine also needs to undergo the same test, but the billions of funds invested in this process make it possible for companies to conduct some tests at the same time, thereby assuming financial risks.
Rino Rappuoli, the chief scientist of the vaccine department of GlaxoSmithKline, said that some public investment and private philanthropists will invest a large amount of money into vaccine companies, and they can do clinical trials. Pre- and Phase I, Phase II and Phase III clinical trials and production will be advanced in parallel rather than in sequence. This means that companies can bet on starting large-scale testing and production of vaccine candidates that may not work.
Campman, director of the London School of Hygiene and Tropical Medicine’s Vaccine Center, said, “This completely reduces the risk of the entire development process.” She said that without the funding, Vaccine science will not produce such fast results. “The Ebola virus has caused devastating damage to Africa, but no more funds have been invested.” The development of the Ebola vaccine took longer. The reason why this money can become cashIn fact, it is because all countries, including rich countries, are facing economic destruction. This also indicates that the development of future vaccines, including the development of vaccines against existing diseases such as malaria, will not be so fast. Rappuoli said, “Unless you invest money, it’s impossible to accelerate.”
Virusologist Peter Hotez of Baylor College of Medicine in Houston, Texas, believes that the motivations of large pharmaceutical companies may be Not only to prevent the spread of the epidemic, but also because the government has the opportunity to fund their research and development. Hotez said that the U.S. “Operation Warp Speed” (Operation Warp Speed) vaccine project has a public investment of approximately US$10 billion, which is “the largest government stimulus package in the history of a pharmaceutical company.”
This driving force does not come entirely from the urgency of the COVID-19 pandemic itself. Past infectious and deadly viruses have prompted the establishment of national and global infrastructure to facilitate faster vaccine development. Graham said that the outbreaks of Ebola and Zika virus marked the beginning of a strengthening of global coordination on how to respond to the infectious disease crisis. He said, “If SARS in 2002 spread like this time, we would not have the current vaccine technology or coordination system, and our life would be difficult.”
In fact, in the final stage of the trial, the new crown epidemic is still prevalent and is also contributing to the further advancement of the vaccine, because companies need cases to prove that the vaccine is effective. Dean, a biostatistician at the University of Florida, said that when the epidemic itself is not epidemic, it is difficult to conduct effectiveness tests, especially in cases such as Middle East Respiratory Syndrome, where the cases are scattered and may reach peaks in some areas, and others. The infection rate in the region is low.
The development experience of the new crown vaccine may also trigger reflections on supervision. Although the strict standards for vaccine approval have not been relaxed, most of the first candidate vaccines were approved under emergency use regulations. These methods are faster, but require the company to conduct follow-up investigations to find side effects and sustained effects. Regulatory agencies of various countries also exchanged information on new coronavirus vaccine trials carried out with the support of the International Alliance of Drug Regulators established in 2012. It aims to reach consensus on some issues, such as