On December 4th, Guangzhou Kangsheng Biotechnology Co., Ltd. registered for registration with the Securities Regulatory Bureau, and the counseling agency was Guangfa Securities.
Editor’s Note: This article comes from WeChat public account ” Know IPO “ (ID: ipoaozhidao), author Uncle C.
According to the news of the IPO, Guangzhou Kangsheng Biotechnology Co., Ltd. registered with the Securities Regulatory Bureau on December 4, and the counseling agency was GF Securities.
Kang Sheng Biological was established in 2001 in Guangzhou Economic and Technological Development Zone, with a registered capital of 94.5 million yuan. It is a biomedical high-tech enterprise established by overseas students studying research and development, production and marketing. At present, the company mainly focuses on research and development, production and marketing of blood purification technology and products.
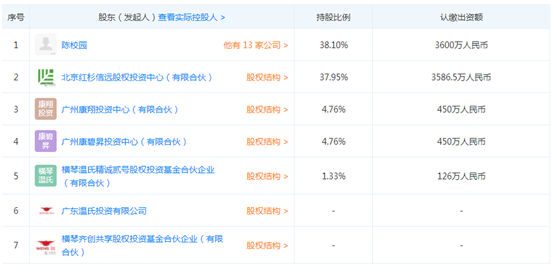
The company’s shareholder information appears in the company’s shareholder information. According to the sky eye inspection, Sequoia Capital subscribed 35.865 million yuan in the name of Beijing Sequoia Xinyuan Equity Investment Center, with a 37.95% shareholding ratio as the company. Second largest shareholder. In addition, China’s largest agricultural and aquaculture company “Wen’s Group” also appeared among the shareholders of Kangsheng Biological, Hengqin Wenshi Jingcheng No. Equity Investment Fund Partnership (Limited Partnership), accounting for 1.26 million yuan in total share capital 1.33% of the shareholders of the partnership, except for Guangdong Wenshi Investment Co., Ltd. (Wenshi Food Group Co., Ltd. wholly-owned), the other shareholders are natural persons.
It is understood that Kangsheng Biological has successfully developed six core products with independent intellectual property rights, including genetically engineered recombinant protein A immunosorbent columns, hemodialysis concentrates, dry hemodialysis powders, hemodialysis online B powders, and dialysis concentrate Centralized preparation and delivery system, medical electric chair, mainly for patients with end-stage renal disease (ESRD), treatment of rheumatic immune disease, desensitization before renal transplantation and postoperative anti-rejection.
The protein A immunoadsorption product is currently the only protein A immunoadsorption product in China that has obtained a registration certificate, which has created a new era in the domestic blood purification clinical treatment field. Because of its wide range of indications, it can be used to treat diseases such as autoimmune diseases and rejection of organ transplantation. It has surpassed similar foreign products, and it has been impossible to solve foreign products.Overcoming the problem of adverse reactions of patients for the first time makes Kangsheng the second in the world and the first company in China to possess this sophisticated technology and products.
The company’s main hemodialysis dry powder and concentrate series products have won praise from users in terms of performance and quality. Its hemodialysis dry powder has won the first patent in this field in China from 2012 to 2014. In just 2 years, sales have doubled, from 5 million units to 10 million units. In terms of the more difficult marketization of high-tech R & D enterprises, the company has also gradually made progress. As early as 2008, “a new type of industrial research on hemodialysis dry powder” won the first prize of scientific and technological achievements in Guangzhou. At present, the company’s sales team accounts for 35% of the total number of people. It has formed a sales network in more than 30 major provinces and cities across the country and serves nearly 1,000 hospitals across the country. It already ranks among the top in the domestic market.
In terms of research and development, two-thirds of the company’s senior technical and management personnel have doctoral degrees / senior titles, and cooperate with relevant domestic universities, research institutions, and hospitals; foreign countries have established technology and product research and development in Europe The center has established extensive and close cooperative relations with relevant European research institutions and enterprises.